Alternating Ring-Opening Metathesis Polymerization (AROMP) of Hydrophobic and Hydrophilic Monomers Provides Oligomers with Side-Chain Sequence Control
- PMID: 30524145
- PMCID: PMC6262599
- DOI: 10.1021/acs.macromol.8b00562
Alternating Ring-Opening Metathesis Polymerization (AROMP) of Hydrophobic and Hydrophilic Monomers Provides Oligomers with Side-Chain Sequence Control
Abstract
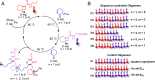
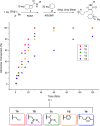
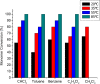
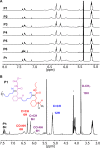
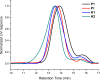
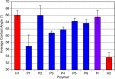
We report the formation of oligomers with side-chain sequence control using ruthenium-catalyzed alternating ring-opening metathesis polymerization (AROMP). These oligomers are prepared through sequential, stoichiometric addition of bicyclo[4.2.0]oct-1(8)-ene-8-carboxamide (monomer A) at 85 °C and cyclohexene (monomer B) at 45 °C to generate sequences up to 24 monomeric units composed of (A-alt- B) n and (A'-alt-B) n microblocks, where n ranges from 1 to 6. Herein, monomer A has an alkyl side chain, and monomer A' has a glycine methyl ester side chain. Increasing microblock size from one to six results in an increasing water contact angle on spin-coated thin films, despite the constant ratio of hydrophilic and hydrophobic moieties. However, a disproportionately high contact angle was observed when n equals 2. Thus, the unique all-carbon backbone formed in the AROMP of bicyclo[4.2.0]oct-1(8)-ene-8-carboxamides and cyclohexene provides a platform for the nontemplated preparation of materials with specific sequences of side chains.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
