A Novel hAPP/htau Mouse Model of Alzheimer's Disease: Inclusion of APP With Tau Exacerbates Behavioral Deficits and Zinc Administration Heightens Tangle Pathology
- PMID: 30524268
- PMCID: PMC6263092
- DOI: 10.3389/fnagi.2018.00382
A Novel hAPP/htau Mouse Model of Alzheimer's Disease: Inclusion of APP With Tau Exacerbates Behavioral Deficits and Zinc Administration Heightens Tangle Pathology
Abstract
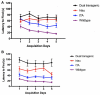
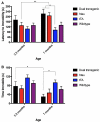
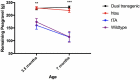
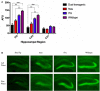
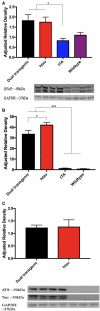
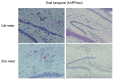
The brains of those with Alzheimer's disease have amyloid and tau pathology; thus, mice modeling AD should have both markers. In this study, we characterize offspring from the cross of the J20 (hAPP) and rTg4510 (htau) strains (referred to as dual Tg). Behavior was assessed at both 3.5 and 7 months, and biochemical differences were assessed at 8 months. Additionally, mice were placed on zinc (Zn) water or standard lab water in order to determine the role of this essential biometal. Behavioral measures examined cognition, emotion, and aspects of daily living. Transgenic mice (dual Tg and htau) showed significant deficits in spatial memory in the Barnes Maze at both 3.5 and 7 months compared to controls. At 7 months, dual Tg mice performed significantly worse than htau mice (p < 0.01). Open field and elevated zero maze (EZM) data indicated that dual Tg and htau mice displayed behavioral disinhibition compared to control mice at both 3.5 and 7 months (p < 0.001). Transgenic mice showed significant deficits in activities of daily living, including burrowing and nesting, at both 3.5 and 7 months compared to control mice (p < 0.01). Dual Tg mice built very poor nests, indicating that non-cognitive tasks are also impacted by AD. Overall, dual Tg mice demonstrated behavioral deficits earlier than those shown by the htau mice. In the brain, dual Tg mice had significantly less free Zn compared to control mice in both the dentate gyrus and the CA3 of the hippocampus (p < 0.01). Dual Tg mice had increased tangles and plaques in the hippocampus compared to htau mice and the dual Tg mice given Zn water displayed increased tangle pathology in the hippocampus compared to htau mice on Zn water (p < 0.05). The dual Tg mouse described here displays pathology reminiscent of the human AD condition and is impaired early on in both cognitive and non-cognitive behaviors. This new mouse model allows researchers to assess how both amyloid and tau in combination impact behavior and brain pathology.
Keywords: Barnes Maze; Zinc; activities of daily living; amyloid; behavior; mouse models; tau.
Figures





References
-
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch. Ophthalmol. 119, 1417–1436. 10.1001/archopht.119.10.1417 - DOI - PMC - PubMed
-
- Alzheimer's Association (2018). Alzheimer's disease facts and figures. Alzheimers Dement. 14, 367–429.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

