GnRH Neurons Provide Direct Input to Hypothalamic Tyrosine Hydroxylase Immunoreactive Neurons Which Is Maintained During Lactation
- PMID: 30524376
- PMCID: PMC6261975
- DOI: 10.3389/fendo.2018.00685
GnRH Neurons Provide Direct Input to Hypothalamic Tyrosine Hydroxylase Immunoreactive Neurons Which Is Maintained During Lactation
Abstract
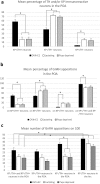
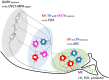
Gonadotropin releasing hormone (GnRH) neurons provide neuronal input to the preoptic area (POA) and the arcuate nucleus (Arc), two regions involved critically in the regulation of neuroendocrine functions and associated behaviors. These areas contain tyrosine hydroxylase immunoreactive (TH-IR) neurons, which play location-specific roles in the neuroendocrine control of both the luteinizing hormone and prolactin secretion, as well as, sexually motivated behaviors. Concerning changes in the activity of GnRH neurons and the secretion pattern of GnRH seen under the influence of rising serum estrogen levels and during lactation, we tested the hypothesis that the functional state of GnRH neurons is mediated via direct synaptic connections to TH-IR neurons in the POA and Arc. In addition, we examined putative changes of these inputs in lactating mice and in mothers separated from their pups. Confocal microscopic and pre-embedding immunohistochemical studies on ovariectomized mice treated with 17β-estradiol (OVX+E2) provided evidence for direct appositions and asymmetric synapses between GnRH-IR fiber varicosities and TH-IR neurons in the POA and the Arc. As TH co-localizes with kisspeptin (KP) in the POA, confocal microscopic analysis was continued on sections additionally labeled for KP. The TH-IR neurons showed a lower level of co-labeling for KP in lactating mice compared to OVX+E2 mice (16.1 ± 5% vs. 57.8 ± 4.3%). Removing the pups for 24 h did not alter significantly the KP production in TH-IR neurons (17.3 ± 4.6%). The mean number of GnRH-IR varicosities on preoptic and arcuate TH cells did not differ in the three animal models investigated. This study shows evidence that GnRH neurons provide direct synaptic inputs to POA and Arc dopaminergic neurons. The scale of anatomical connectivity with these target cells was unaltered during lactation indicating a maintained GnRH input, inspite of the altered hormonal condition.
Keywords: arcuate nucleus; asymmetric synapses; estrogen; kisspeptin; lactation; preoptic area; tyrosine hydroxylase.
Figures




References
-
- Leranth C, Segura LM, Palkovits M, MacLusky NJ, Shanabrough M, Naftolin F. The LH-RH-containing neuronal network in the preoptic area of the rat: demonstration of LH-RH-containing nerve terminals in synaptic contact with LH-RH neurons. Brain Res. (1985) 345:332–6. 10.1016/0006-8993(85)91011-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

