Baseline Gastrointestinal Eosinophilia Is Common in Oral Immunotherapy Subjects With IgE-Mediated Peanut Allergy
- PMID: 30524424
- PMCID: PMC6261984
- DOI: 10.3389/fimmu.2018.02624
Baseline Gastrointestinal Eosinophilia Is Common in Oral Immunotherapy Subjects With IgE-Mediated Peanut Allergy
Abstract
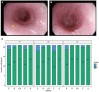
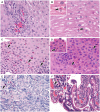
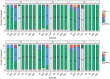
Rationale: Oral immunotherapy (OIT) is an emerging treatment for food allergy. While desensitization is achieved in most subjects, many experience gastrointestinal symptoms and few develop eosinophilic gastrointestinal disease. It is unclear whether these subjects have subclinical gastrointestinal eosinophilia (GE) at baseline. We aimed to evaluate the presence of GE in subjects with food allergy before peanut OIT. Methods: We performed baseline esophagogastroduodenoscopies on 21 adults before undergoing peanut OIT. Subjects completed a detailed gastrointestinal symptom questionnaire. Endoscopic findings were assessed using the Eosinophilic Esophagitis (EoE) Endoscopic Reference Score (EREFS) and biopsies were obtained from the esophagus, gastric antrum, and duodenum. Esophageal biopsies were evaluated using the EoE Histologic Scoring System. Immunohistochemical staining for eosinophil peroxidase (EPX) was also performed. Hematoxylin and eosin and EPX stains of each biopsy were assessed for eosinophil density and EPX/mm2 was quantified using automated image analysis. Results: All subjects were asymptomatic. Pre-existing esophageal eosinophilia (>5 eosinophils per high-power field [eos/hpf]) was present in five participants (24%), three (14%) of whom had >15 eos/hpf associated with mild endoscopic findings (edema, linear furrowing, or rings; median EREFS = 0, IQR 0-0.25). Some subjects also demonstrated basal cell hyperplasia, dilated intercellular spaces, and lamina propria fibrosis. Increased eosinophils were noted in the gastric antrum (>12 eos/hpf) or duodenum (>26 eos/hpf) in 9 subjects (43%). EPX/mm2 correlated strongly with eosinophil counts (r = 0.71, p < 0.0001). Conclusions: Pre-existing GE is common in adults with IgE-mediated peanut allergy. Eosinophilic inflammation (EI) in these subjects may be accompanied by mild endoscopic and histologic findings. Longitudinal data collection during OIT is ongoing.
Keywords: Eosinophilic Esophagitis; adverse event; biopsy; endoscopy; eosinophil; gastrointestinal; oral immunotherapy; peanut food allergy.
Figures



References
-
- Venkataraman D, Erlewyn-Lajeunesse M, Kurukulaaratchy RJ, Potter S, Roberts G, Matthews S, et al. . Prevalence and longitudinal trends of food allergy during childhood and adolescence: results of the isle of wight birth cohort study. Clin Exp Allergy (2018) 48:394–402. 10.1111/cea.13088 - DOI - PMC - PubMed
-
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. . Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. (2011) 127:668–76 e1-2. 10.1016/j.jaci.2011.01.039 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

