Rice Calcineurin B-Like Protein-Interacting Protein Kinase 31 (OsCIPK31) Is Involved in the Development of Panicle Apical Spikelets
- PMID: 30524455
- PMCID: PMC6262370
- DOI: 10.3389/fpls.2018.01661
Rice Calcineurin B-Like Protein-Interacting Protein Kinase 31 (OsCIPK31) Is Involved in the Development of Panicle Apical Spikelets
Abstract
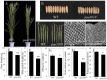
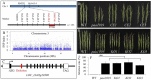
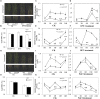
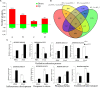
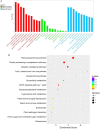
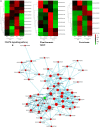
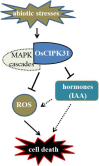
Panicle apical abortion (PAA) causes severe yield losses in rice production, but details about its development and molecular basis remain elusive. Herein, a PAA mutant, paa1019, was identified among the progeny of an elite indica maintainer rice line Yixiang 1B (YXB) mutagenized population obtained using ethyl methyl sulfonate. The abortion rate of spikelets in paa1019 was observed up to 60%. Genetic mapping combined with Mutmap analysis revealed that LOC_Os03g20380 harbored a single-bp substitution (C to T) that altered its transcript length. This gene encodes calcineurin B-like protein-interacting protein kinase 31 (OsCIPK31) localized into the cytoplasm, and is preferentially expressed in transport tissues of rice. Complementation of paa1019 by transferring the open reading frame of LOC_Os03g20380 from YXB reversed the mutant phenotype, and conversely, gene editing by knocking out of OsCIPK31 in YXB results in PAA phenotype. Our results support that OsCIPK31 plays an important role in panicle development. We found that dysregulation is caused by the disruption of OsCIPK31 function due to excessive accumulation of ROS, which ultimately leads to cell death in rice panicle. OsCIPK31 and MAPK pathway might have a synergistic effect to lead ROS accumulation in response to stresses. Meanwhile the PAA distribution is related to IAA hormone accumulation in the panicle. Our study provides an understanding of the role of OsCIPK31 in panicle development by responding to various stresses and phytohormones.
Keywords: CIPK; IAA; MAPK signaling; ROS; apical dominance; auxin; panicle apical abortion.
Figures



References
-
- Ansari T. H., Yamamoto Y., Yoshida T., Miyazaki A., Wang Y. (2003). Cultivar differences in the number of differentiated spikelets and percentage of degenerated spikelets as determinants of the spikelet number per panicle in relation to dry matter production and nitrogen absorption. Soil Sci. Plant Nutr. 49 433–444. 10.1080/00380768.2003.10410029 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

