Dendritic cell-based vaccination: powerful resources of immature dendritic cells against pancreatic adenocarcinoma
- PMID: 30524902
- PMCID: PMC6279335
- DOI: 10.1080/2162402X.2018.1504727
Dendritic cell-based vaccination: powerful resources of immature dendritic cells against pancreatic adenocarcinoma
Abstract
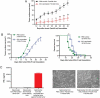
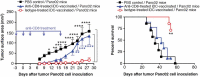
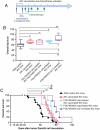
Pancreatic adenocarcinoma (PAC) has a poor prognosis. One treatment approach, investigated here, is to reinforce antitumor immunity. Dendritic cells (DCs) are essential for the development and regulation of adaptive host immune responses against tumors. A major role for DCs may be as innate tumoricidal effector cells. We explored the efficacy of vaccination with immature (i)DCs, after selecting optimal conditions for generating immunostimulatory iDCs. We used two models, C57BL/6Jrj mice with ectopic tumors induced by the PAC cell line, Panc02, and genetically engineered (KIC) mice developing PAC. Therapeutic iDC-vaccination resulted in a significant reduction in tumor growth in C57BL/6Jrj mice and prolonged survival in KIC mice. Prophylactic iDC-vaccination prevented subcutaneous tumor development. These protective effects were long-lasting in Panc02-induced tumor development, but not in melanoma. iDC-vaccination impacted the immune status of the hosts by greatly increasing the percentage of CD8+ T-cells, and natural killer (NK)1.1+ cells, that express granzyme B associated with Lamp-1 and IFN-γ. Efficacy of iDC-vaccination was CD8+ T-cell-dependent but NK1.1+ cell-independent. We demonstrated the ability of DCs to produce peroxynitrites and to kill tumor cells; this killing activity involved peroxynitrites. Altogether, these findings make killer DCs the pivotal actors in the beneficial clinical outcome that accompanies antitumor immune responses. We asked whether efficacy can be improved by combining DC-vaccination with the FOLFIRINOX regimen. Combined treatment significantly increased the lifespan of KIC mice with PAC. Prolonged treatment with FOLFIRINOX clearly augmented this beneficial effect. Combining iDC-vaccination with FOLFIRINOX may therefore represent a promising therapeutic option for patients with PAC.
Keywords: Active immunotherapy; DC-based tumor immunotherapy; FOLFIRINOX; animal models; cancer vaccines; pancreatic cancer.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
