Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival
- PMID: 30524905
- PMCID: PMC6279322
- DOI: 10.1080/2162402X.2018.1509820
Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival
Abstract
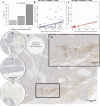
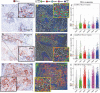
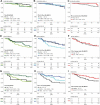
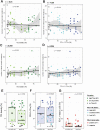
To better understand the expression pattern of programmed death-ligand 1 (PD-L1) expression in different breast cancer types, we characterized PD-L1 expression in tumor and tumor-infiltrating immune cells, in relation to mutation rate, BRCA1-like status and survival. We analyzed 410 primary treatment-naive breast tumors comprising 162 estrogen receptor-positive (ER+) and HER2-, 101 HER2+ and 147 triple-negative (TN) cancers. Pathologists quantified tumor-infiltrating lymphocytes (TILs) and PD-L1 expression in tumor cells and TILs using whole slides and tissue microarray. Mutation rate was assessed by DNA sequencing, BRCA1-like status using multiplex ligation-dependent probe amplification, and immune landscape by multiplex image analyses of CD4, CD68, CD8, FOXP3, cytokeratin, and PD-L1. Half of PD-L1 scores evaluated by tissue microarray were false negatives compared to whole slide evaluations. We observed at least 1% of PD-L1-positive (PD-L1+) cells in 53.1% of ER+HER2-, 73.3% of HER2+, and 84.4% of TN tumors. PD-L1 expression was higher in ductal compared to lobular carcinomas, also within ER+HER2- tumors (p = 0.04). High PD-L1+ TILs score (> 50%) was independently associated with better outcome in TN tumors (HR = 0.27; 95%CI = 0.10-0.69). Within TN tumors, PD-L1 and TIL scores showed a modest but significant positive association with the number of silent mutations, but no association with BRCA1-like status. Multiplex image analyses indicated that PD-L1 is expressed on multiple immune cells (CD68+ macrophages, CD4+, FOXP3+, and CD8+ T cells) in the breast tumor microenvironment, independent of the PD-L1 status of the tumor cells. We found no evidence that levels of PD-L1+ TILs in TN breast cancer are driven by high mutation rate or BRCA1-like status.
Keywords: BRCA1-like; Breast cancer; PD-L1; TILs; mutations.
Figures


References
-
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of Experimental Medicine. 2000;192:10.1084/jem.192.7.102727–34 PMID:11015443. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
