Stuffed Methyltransferase Catalyzes the Penultimate Step of Pyochelin Biosynthesis
- PMID: 30525512
- PMCID: PMC6372355
- DOI: 10.1021/acs.biochem.8b00716
Stuffed Methyltransferase Catalyzes the Penultimate Step of Pyochelin Biosynthesis
Abstract
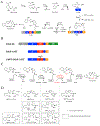
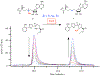
Nonribosomal peptide synthetases use tailoring domains to incorporate chemical diversity into the final natural product. A structurally unique set of tailoring domains are found to be stuffed within adenylation domains and have only recently begun to be characterized. PchF is the NRPS termination module in pyochelin biosynthesis and includes a stuffed methyltransferase domain responsible for S-adenosylmethionine (AdoMet)-dependent N-methylation. Recent studies of stuffed methyltransferase domains propose a model in which methylation occurs on amino acids after adenylation and thiolation rather than after condensation to the nascent peptide chain. Herein, we characterize the adenylation and stuffed methyltransferase didomain of PchF through the synthesis and use of substrate analogues, steady-state kinetics, and onium chalcogen effects. We provide evidence that methylation occurs through an SN2 reaction after thiolation, condensation, cyclization, and reduction of the module substrate cysteine and is the penultimate step in pyochelin biosynthesis.
Figures
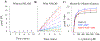
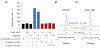
Similar articles
-
In vitro reconstitution of the Pseudomonas aeruginosa nonribosomal peptide synthesis of pyochelin: characterization of backbone tailoring thiazoline reductase and N-methyltransferase activities.Biochemistry. 2001 Jul 31;40(30):9023-31. doi: 10.1021/bi010519n. Biochemistry. 2001. PMID: 11467965
-
Unimodular Methylation by Adenylation-Thiolation Domains Containing an Embedded Methyltransferase.J Mol Biol. 2020 Oct 2;432(21):5802-5808. doi: 10.1016/j.jmb.2020.09.004. Epub 2020 Sep 11. J Mol Biol. 2020. PMID: 32920052
-
Assembly of the Pseudomonas aeruginosa nonribosomal peptide siderophore pyochelin: In vitro reconstitution of aryl-4, 2-bisthiazoline synthetase activity from PchD, PchE, and PchF.Biochemistry. 1999 Nov 9;38(45):14941-54. doi: 10.1021/bi991787c. Biochemistry. 1999. PMID: 10555976
-
Nonribosomal peptides for iron acquisition: pyochelin biosynthesis as a case study.Curr Opin Struct Biol. 2018 Dec;53:1-11. doi: 10.1016/j.sbi.2018.01.015. Epub 2018 Feb 20. Curr Opin Struct Biol. 2018. PMID: 29455106 Free PMC article. Review.
-
[Advances in the study of the mechanism and application of nonribosomal peptide synthetases].Wei Sheng Wu Xue Bao. 2007 Aug;47(4):734-7. Wei Sheng Wu Xue Bao. 2007. PMID: 17944384 Review. Chinese.
Cited by
-
Expanding the substrate selectivity of the fimsbactin biosynthetic adenylation domain, FbsH.bioRxiv [Preprint]. 2024 Jul 26:2024.07.26.605314. doi: 10.1101/2024.07.26.605314. bioRxiv. 2024. Update in: ACS Chem Biol. 2024 Dec 20;19(12):2451-2461. doi: 10.1021/acschembio.4c00512. PMID: 39091846 Free PMC article. Updated. Preprint.
-
Relationship between Pyochelin and Pseudomonas Quinolone Signal in Pseudomonas aeruginosa: A Direction for Future Research.Int J Mol Sci. 2024 Aug 7;25(16):8611. doi: 10.3390/ijms25168611. Int J Mol Sci. 2024. PMID: 39201297 Free PMC article. Review.
-
Structural advances toward understanding the catalytic activity and conformational dynamics of modular nonribosomal peptide synthetases.Nat Prod Rep. 2023 Sep 20;40(9):1550-1582. doi: 10.1039/d3np00003f. Nat Prod Rep. 2023. PMID: 37114973 Free PMC article. Review.
-
Unique Initiation and Termination Mechanisms Involved in the Biosynthesis of a Hybrid Polyketide-Nonribosomal Peptide Lyngbyapeptin B Produced by the Marine Cyanobacterium Moorena bouillonii.ACS Chem Biol. 2023 Apr 21;18(4):875-883. doi: 10.1021/acschembio.3c00011. Epub 2023 Mar 15. ACS Chem Biol. 2023. PMID: 36921345 Free PMC article.
-
Expanding the Substrate Selectivity of the Fimsbactin Biosynthetic Adenylation Domain, FbsH.ACS Chem Biol. 2024 Dec 20;19(12):2451-2461. doi: 10.1021/acschembio.4c00512. Epub 2024 Nov 8. ACS Chem Biol. 2024. PMID: 39513969
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

