Role of Biologics in Asthma
- PMID: 30525902
- PMCID: PMC6835092
- DOI: 10.1164/rccm.201810-1944CI
Role of Biologics in Asthma
Abstract
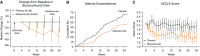
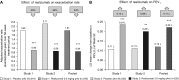
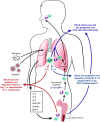
Patients with severe uncontrolled asthma have disproportionally high morbidity and healthcare utilization as compared with their peers with well-controlled disease. Although treatment options for these patients were previously limited, with unacceptable side effects, the emergence of biologic therapies for the treatment of asthma has provided promising targeted therapy for these patients. Biologic therapies target specific inflammatory pathways involved in the pathogenesis of asthma, particularly in patients with an endotype driven by type 2 (T2) inflammation. In addition to anti-IgE therapy that has improved outcomes in allergic asthma for more than a decade, three anti-IL-5 biologics and one anti-IL-4R biologic have recently emerged as promising treatments for T2 asthma. These targeted therapies have been shown to reduce asthma exacerbations, improve lung function, reduce oral corticosteroid use, and improve quality of life in appropriately selected patients. In addition to the currently approved biologic agents, several biologics targeting upstream inflammatory mediators are in clinical trials, with possible approval on the horizon. This article reviews the mechanism of action, indications, expected benefits, and side effects of each of the currently approved biologics for severe uncontrolled asthma and discusses promising therapeutic targets for the future.
Keywords: asthma treatments; biologics; eosinophils; monoclonal antibodies; severe asthma.
Figures




Comment in
-
Selection of Biologics for Type 2-High Asthma.Am J Respir Crit Care Med. 2019 Sep 15;200(6):790-791. doi: 10.1164/rccm.201904-0763LE. Am J Respir Crit Care Med. 2019. PMID: 31106563 Free PMC article. No abstract available.
-
Reply to Yilmaz: Selection of Biologics for Type 2-High Asthma.Am J Respir Crit Care Med. 2019 Sep 15;200(6):791-792. doi: 10.1164/rccm.201904-0898LE. Am J Respir Crit Care Med. 2019. PMID: 31106571 Free PMC article. No abstract available.
References
-
- Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. - PMC - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma Eur Respir J 201443343–373.[Published erratum appears in Eur Respir J 52:1352020.] - PubMed
-
- Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–729. - PubMed
-
- Hyland ME, Whalley B, Jones RC, Masoli M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res. 2015;24:631–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

