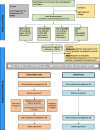
Study protocol of a 4- parallel arm, superiority, community based cluster randomized controlled trial comparing paper and e-platform based interventions to improve accuracy of recall of last menstrual period (LMP) dates in rural Bangladesh
- PMID: 30526560
- PMCID: PMC6288958
- DOI: 10.1186/s12889-018-6258-z
Study protocol of a 4- parallel arm, superiority, community based cluster randomized controlled trial comparing paper and e-platform based interventions to improve accuracy of recall of last menstrual period (LMP) dates in rural Bangladesh
Abstract
Background: Gestational age (GA) is a key determinant of newborn survival and long-term impairment. Accurate estimation of GA facilitates timely provision of essential interventions to improve maternal and newborn outcomes. Menstrual based dating, ultrasound based dating, and neonatal estimates are the primarily used methods for assessing GA; all of which have some strength and weaknesses that require critical consideration. Last menstrual period (LMP) is simple, low-cost self-reported information, recommended by the World Health Organization for estimating GA but has issues of recall mainly among poorer, less educated women and women with irregular menstruation, undiagnosed abortion, and spotting during early pregnancy. Several studies have noted that about 20-50% of women cannot accurately recall the date of LMP. The goal of this study is therefore to improve recall and reporting of LMP and by doing so increase the accuracy of LMP based GA assessment in a rural population of Bangladesh where antenatal care-seeking, availability and utilization of USG is low.
Method: We propose to conduct a 4- parallel arm, superiority, community based cluster randomized controlled trial comparing three interventions to improve recall of GA with a no intervention arm. The interventions include (i) counselling and a paper based calendar (ii) counselling and a cell phone based SMS alert system (iii) counselling and smart-phone application. The trial is being conducted among 3360 adolescent girls and recently married women in Mirzapur sub-district of Bangladesh.
Discussion: Enrolment of study participants continued from January 24, 2017 to March 29, 2017. Data collection and intervention implementation is ongoing and will end by February, 2019. Data analysis will measure efficacy of interventions in improving the recall of LMP date among enrolled participants. Results will be reported following CONSORT guideline. The innovative conventional & e-platform based interventions, if successful, can provide substantial evidence to scale-up in a low resource setting where m-Health initiatives are proliferating with active support from all sectors in policy and implementation.
Trial registration: ClinicalTrials.gov NCT02944747 . The trial has been registered before starting enrolment on 24 October 2016.
Keywords: Bangladesh; Gestational age; LMP; M-health; Mobile phone; Preterm birth; Recall.
Conflict of interest statement
Ethics approval and consent to participate
The trial is being conducted following the ethical principles in the Declaration of Helsinki and good practice guidelines on the proper conduct of research. Institutional review board of icddr,b is the regulatory body for monitoring the progress of the trial. The trial has been reviewed and approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of the Institutional Review Board of icddr,b (PR16031). Before staring enrolment of study participants, this trial has been registered with
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International . Bangladesh Demographic and Health Survey 2014: Key Indicators. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International; 2015.
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical