Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes
- PMID: 30526677
- PMCID: PMC6286508
- DOI: 10.1186/s13287-018-1085-9
Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes
Abstract
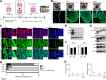
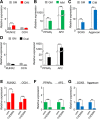
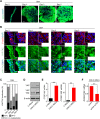
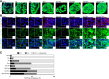
Background: Three-dimensional (3D) floating culture clumps of mesenchymal stem cell (MSC)/extracellular matrix (ECM) complexes (C-MSCs) consist of cells and self-produced ECM. Previous studies have demonstrated that C-MSCs can be transplanted into bony lesions without an artificial scaffold to induce bone regeneration. Moreover, osteoinductive medium (OIM)-treated C-MSCs (OIM-C-MSCs) have shown rapid and increased new bone formation in vivo. To apply OIM-C-MSCs for novel bone regenerative cell therapy, their cellular properties at the molecular level must be elucidated. The transcriptional co-activators yes-associated protein/transcriptional co-activator with PDZ-binding motif (YAP/TAZ) have been recognized as key players in the mechanotransduction cascade, controlling cell lineage commitment in MSCs. It is plausible that 3D C-MSCs/OIM-C-MSCs cultured in floating conditions could provide distinct microenvironments compared to conventional 2D culture systems and thereby induce unique mechanotransduction cascades. Therefore, this study investigated the YAP/TAZ activity in 3D-cultured C-MSCs/OIM-C-MSCs in floating conditions.
Methods: Human bone marrow-derived MSCs were cultured in growth medium supplemented with ascorbic acid. To obtain C-MSCs, confluent cells that had formed on the cellular sheet were scratched using a micropipette tip and were then torn off. The sheet was rolled to make round clumps of cells. Then, YAP/TAZ activity, filamentous actin (F-actin) integrity, collagen type I (COL1) production, and the differentiation potency in 3D floating culture C-MSCs/OIM-C-MSCs were analyzed.
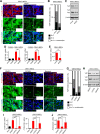
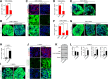
Results: C-MSCs cultured in floating conditions lost their actin cytoskeleton to downregulate YAP/TAZ activity, which directed cells to undergo adipogenesis/chondrogenesis. OIM treatment induced abundant COL1 deposition, which facilitated Intβ1-dependent actin fiber formation and YAP/TAZ activity to elevate the expression levels of osteogenic master transcriptional factor runt-related transcription factor 2 (RUNX2) mRNA in C-MSCs. Importantly, elevation of YAP/TAZ activity via OIM was associated with COL1 deposition and F-actin integrity, suggesting a positive feedback loop in OIM-C-MSCs.
Conclusion: These findings suggest that OIM-C-MSCs, which form a unique microenvironment that maintains high YAP/TAZ activity, can serve as better candidates for bone regenerative cell therapy than C-MSCs.
Keywords: 3D culture; C-MSCs; F-actin; Mechanotransduction; YAP/TAZ.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for human subjects was obtained from the Independent Ethics Committee of Hiroshima University (reference number 422-2).
Consent for publication
Not applicable. This manuscript does not contain any individual person’s data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Cryopreserved clumps of mesenchymal stem cell/extracellular matrix complexes retain osteogenic capacity and induce bone regeneration.Stem Cell Res Ther. 2018 Mar 21;9(1):73. doi: 10.1186/s13287-018-0826-0. Stem Cell Res Ther. 2018. PMID: 29562931 Free PMC article.
-
MSC/ECM Cellular Complexes Induce Periodontal Tissue Regeneration.J Dent Res. 2017 Aug;96(9):984-991. doi: 10.1177/0022034517708770. Epub 2017 May 18. J Dent Res. 2017. PMID: 28521114
-
Role of YAP/TAZ in mechanotransduction.Nature. 2011 Jun 8;474(7350):179-83. doi: 10.1038/nature10137. Nature. 2011. PMID: 21654799
-
[Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP].Yi Chuan. 2013 Nov;35(11):1283-90. doi: 10.3724/sp.j.1005.2013.01283. Yi Chuan. 2013. PMID: 24579311 Review. Chinese.
-
Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells.Tissue Eng Part B Rev. 2020 Feb;26(1):13-25. doi: 10.1089/ten.TEB.2019.0250. Epub 2019 Nov 26. Tissue Eng Part B Rev. 2020. PMID: 31663422 Review.
Cited by
-
Additively manufactured bioceramic scaffolds based on triply periodic minimal surfaces for bone regeneration.J Tissue Eng. 2024 Apr 11;15:20417314241244997. doi: 10.1177/20417314241244997. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38617462 Free PMC article.
-
Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis.Int J Mol Sci. 2019 Aug 15;20(16):3970. doi: 10.3390/ijms20163970. Int J Mol Sci. 2019. PMID: 31443173 Free PMC article.
-
The physiological and pathogenic roles of yes-associated protein/transcriptional co-activator with PDZ-binding motif in bone or skeletal motor system-related cells.Cytojournal. 2025 Feb 8;22:13. doi: 10.25259/Cytojournal_237_2024. eCollection 2025. Cytojournal. 2025. PMID: 40134564 Free PMC article. Review.
-
MRCK as a Potential Target for Claudin-Low Subtype of Breast Cancer.Int J Biol Sci. 2024 Jan 1;20(1):1-14. doi: 10.7150/ijbs.88285. eCollection 2024. Int J Biol Sci. 2024. PMID: 38164185 Free PMC article.
-
Role of YAP/TAZ in bone diseases: A transductor from mechanics to biology.J Orthop Translat. 2025 Jan 20;51:13-23. doi: 10.1016/j.jot.2024.12.003. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 39902099 Free PMC article. Review.
References
-
- Nagahara T, Yoshimatsu S, Shiba H, Kawaguchi H, Takeda K, Iwata T, Mizuno N, Fujita T, Kurihara H. Introduction of a mixture of beta-tricalcium phosphate into a complex of bone marrow mesenchymal stem cells and type I collagen can augment the volume of alveolar bone without impairing cementum regeneration. J Periodontol. 2015;86(3):456–464. doi: 10.1902/jop.2014.140384. - DOI - PubMed
-
- Kittaka M, Kajiya M, Shiba H, Takewaki M, Takeshita K, Khung R, Fujita T, Iwata T, Nguyen TQ, Ouhara K, et al. Clumps of a mesenchymal stromal cell/extracellular matrix complex can be a novel tissue engineering therapy for bone regeneration. Cytotherapy. 2015;17(7):860–873. doi: 10.1016/j.jcyt.2015.01.007. - DOI - PubMed
-
- Takeshita K, Motoike S, Kajiya M, Komatsu N, Takewaki M, Ouhara K, Iwata T, Takeda K, Mizuno N, Fujita T, et al. Xenotransplantation of interferon-gamma-pretreated clumps of a human mesenchymal stem cell/extracellular matrix complex induces mouse calvarial bone regeneration. Stem Cell Res Ther. 2017;8(1):101. doi: 10.1186/s13287-017-0550-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JP17K173510A/Japan Society for the Promotion of Science/International
- JP17H06897/Japan Society for the Promotion of Science/International
- JP18H02977/Japan Society for the Promotion of Science/International
- Research Grant/Nakatomi Foundation/International
- Research Grant/Mitsui Sumitomo Insurance Welfare Foundation/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

