The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat
- PMID: 30527629
- PMCID: PMC6440809
- DOI: 10.1016/j.biopsych.2018.10.009
The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat
Abstract
Background: Stress is associated with an increased prevalence of anxiety and depression. Repeated social defeat (RSD) stress in mice increases the release of monocytes from the bone marrow that are recruited to the brain by microglia. These monocytes enhance inflammatory signaling and augment anxiety. Moreover, RSD promotes stress sensitization, in which exposure to acute stress 24 days after cessation of RSD causes anxiety recurrence. The purpose of this study was to determine whether microglia were critical to stress sensitization and exhibited increased reactivity to subsequent acute stress or immune challenge.
Methods: Mice were exposed to RSD, microglia were eliminated by colony-stimulating factor 1 receptor antagonism (PLX5622) and allowed to repopulate, and responses to acute stress or immune challenge (lipopolysaccharide) were determined 24 days after RSD sensitization.
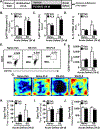
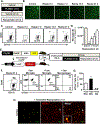
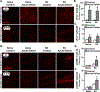
Results: Microglia maintained a unique messenger RNA signature 24 days after RSD. Moreover, elimination of RSD-sensitized microglia prevented monocyte accumulation in the brain and blocked anxiety recurrence following acute stress (24 days). When microglia were eliminated prior to RSD and repopulated and mice were subjected to acute stress, there was monocyte accumulation in the brain and anxiety in RSD-sensitized mice. These responses were unaffected by microglial elimination/repopulation. This may be related to neuronal sensitization that persisted 24 days after RSD. Following immune challenge, there was robust microglial reactivity in RSD-sensitized mice associated with prolonged sickness behavior. Here, microglial elimination/repopulation prevented the amplified immune reactivity ex vivo and in vivo in RSD-sensitized mice.
Conclusions: Microglia and neurons remain sensitized weeks after RSD, and only the immune reactivity component of RSD-sensitized microglia was prevented by elimination/repopulation.
Keywords: Anxiety; CSF1R antagonist; Microglia; Monocytes; Repeated social defeat; Stress.
Copyright © 2018 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures




Comment in
-
Microglia, Monocytes, and the Recurrence of Anxiety in Stress-Sensitized Mice.Biol Psychiatry. 2019 Jun 15;85(12):e67-e68. doi: 10.1016/j.biopsych.2018.11.027. Epub 2019 Mar 8. Biol Psychiatry. 2019. PMID: 30857640 No abstract available.
-
Reply to: Microglia, Monocytes, and the Recurrence of Anxiety in Stress-Sensitized Mice.Biol Psychiatry. 2019 Jun 15;85(12):e69-e70. doi: 10.1016/j.biopsych.2019.01.026. Epub 2019 Mar 8. Biol Psychiatry. 2019. PMID: 30857641 No abstract available.
-
From Stress Sensitization to Microglial Priming and Vice Versa: A New Era of Research in Biological Psychiatry.Biol Psychiatry. 2019 Apr 15;85(8):619-620. doi: 10.1016/j.biopsych.2019.02.002. Biol Psychiatry. 2019. PMID: 30922465 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

