Single-Neuron Correlates of Error Monitoring and Post-Error Adjustments in Human Medial Frontal Cortex
- PMID: 30528064
- PMCID: PMC6354767
- DOI: 10.1016/j.neuron.2018.11.016
Single-Neuron Correlates of Error Monitoring and Post-Error Adjustments in Human Medial Frontal Cortex
Abstract
Humans can self-monitor errors without explicit feedback, resulting in behavioral adjustments on subsequent trials such as post-error slowing (PES). The error-related negativity (ERN) is a well-established macroscopic scalp EEG correlate of error self-monitoring, but its neural origins and relationship to PES remain unknown. We recorded in the frontal cortex of patients performing a Stroop task and found neurons that track self-monitored errors and error history in dorsal anterior cingulate cortex (dACC) and pre-supplementary motor area (pre-SMA). Both the intracranial ERN (iERN) and error neuron responses appeared first in pre-SMA, and ∼50 ms later in dACC. Error neuron responses were correlated with iERN amplitude on individual trials. In dACC, such error neuron-iERN synchrony and responses of error-history neurons predicted the magnitude of PES. These data reveal a human single-neuron correlate of the ERN and suggest that dACC synthesizes error information to recruit behavioral control through coordinated neural activity.
Keywords: anterior cingulate cortex; cognitive control; error monitoring; executive function; human intracranial; human single-neuron; medial frontal cortex; post-error slowing; pre-supplementary motor area.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
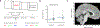
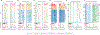
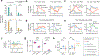

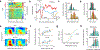
Similar articles
-
Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing.J Neurosci. 2010 Sep 22;30(38):12759-69. doi: 10.1523/JNEUROSCI.3274-10.2010. J Neurosci. 2010. PMID: 20861380 Free PMC article.
-
Error detection in patients with lesions to the medial prefrontal cortex: an ERP study.Neuropsychologia. 2004;42(1):118-30. doi: 10.1016/s0028-3932(03)00121-0. Neuropsychologia. 2004. PMID: 14615082 Clinical Trial.
-
Conflict monitoring and error processing: new insights from simultaneous EEG-fMRI.Neuroimage. 2015 Jan 15;105:395-407. doi: 10.1016/j.neuroimage.2014.10.028. Epub 2014 Oct 22. Neuroimage. 2015. PMID: 25462691
-
Meta-analysis of aberrant post-error slowing in substance use disorder: implications for behavioral adaptation and self-control.Eur J Neurosci. 2019 Aug;50(3):2467-2476. doi: 10.1111/ejn.14229. Epub 2018 Dec 3. Eur J Neurosci. 2019. PMID: 30383336 Free PMC article. Review.
-
Neurophysiological mechanisms of error monitoring in human and non-human primates.Nat Rev Neurosci. 2023 Mar;24(3):153-172. doi: 10.1038/s41583-022-00670-w. Epub 2023 Jan 27. Nat Rev Neurosci. 2023. PMID: 36707544 Free PMC article. Review.
Cited by
-
EEG correlates of physical effort and reward processing during reinforcement learning.J Neurophysiol. 2020 Aug 1;124(2):610-622. doi: 10.1152/jn.00370.2020. Epub 2020 Jul 29. J Neurophysiol. 2020. PMID: 32727262 Free PMC article.
-
Long-term effects of repeated multitarget high-definition transcranial direct current stimulation combined with cognitive training on response inhibition gains.Front Neurosci. 2023 Mar 9;17:1107116. doi: 10.3389/fnins.2023.1107116. eCollection 2023. Front Neurosci. 2023. PMID: 36968503 Free PMC article.
-
Testing Models of Human Declarative Memory at the Single-Neuron Level.Trends Cogn Sci. 2019 Jun;23(6):510-524. doi: 10.1016/j.tics.2019.03.006. Epub 2019 Apr 25. Trends Cogn Sci. 2019. PMID: 31031021 Free PMC article. Review.
-
Error monitoring when no errors are possible: Arbitrary free-choice decisions invoke error monitoring processes.iScience. 2023 Mar 10;26(4):106373. doi: 10.1016/j.isci.2023.106373. eCollection 2023 Apr 21. iScience. 2023. PMID: 37009217 Free PMC article.
-
On the Comparison Between the Nc/CRN and the Ne/ERN.Front Hum Neurosci. 2022 Jun 23;15:788167. doi: 10.3389/fnhum.2021.788167. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35812306 Free PMC article. Review.
References
-
- Aarts E, Verhage M, Veenvliet JV, Dolan CV & van der Sluis S 2014. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci, 17, 491–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

