Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex
- PMID: 30528065
- PMCID: PMC6344231
- DOI: 10.1016/j.neuron.2018.11.021
Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex
Abstract
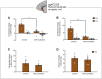
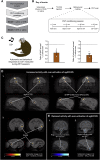
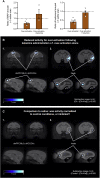
Anhedonia is a core symptom of depression, but the underlying neurobiological mechanisms are unknown. Correlative neuroimaging studies implicate dysfunction within ventromedial prefrontal cortex, but the causal roles of specific subregions remain unidentified. We addressed these issues by combining intracerebral microinfusions with cardiovascular and behavioral monitoring in marmoset monkeys to show that over-activation of primate subgenual anterior cingulate cortex (sgACC, area 25) blunts appetitive anticipatory, but not consummatory, arousal, whereas manipulations of adjacent perigenual ACC (pgACC, area 32) have no effect. sgACC/25 over-activation also reduces the willingness to work for reward. 18F-FDG PET imaging reveals over-activation induced metabolic changes in circuits involved in reward processing and interoception. Ketamine treatment ameliorates the blunted anticipatory arousal and reverses associated metabolic changes. These results demonstrate a causal role for primate sgACC/25 over-activity in selective aspects of impaired reward processing translationally relevant to anhedonia, and ketamine's modulation of an affective network to exert its action.
Keywords: PET imaging; anhedonia; anterior cingulate; area 25; depression; ketamine; marmoset; motivation; reward; subgenual.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Amat J., Baratta M.V., Paul E., Bland S.T., Watkins L.R., Maier S.F. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

