Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment
- PMID: 30528430
- PMCID: PMC6329664
- DOI: 10.1016/j.cell.2018.10.049
Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment
Abstract
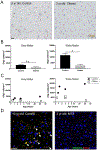
Chemotherapy results in a frequent yet poorly understood syndrome of long-term neurological deficits. Neural precursor cell dysfunction and white matter dysfunction are thought to contribute to this debilitating syndrome. Here, we demonstrate persistent depletion of oligodendrocyte lineage cells in humans who received chemotherapy. Developing a mouse model of methotrexate chemotherapy-induced neurological dysfunction, we find a similar depletion of white matter OPCs, increased but incomplete OPC differentiation, and a persistent deficit in myelination. OPCs from chemotherapy-naive mice similarly exhibit increased differentiation when transplanted into the microenvironment of previously methotrexate-exposed brains, indicating an underlying microenvironmental perturbation. Methotrexate results in persistent activation of microglia and subsequent astrocyte activation that is dependent on inflammatory microglia. Microglial depletion normalizes oligodendroglial lineage dynamics, myelin microstructure, and cognitive behavior after methotrexate chemotherapy. These findings indicate that methotrexate chemotherapy exposure is associated with persistent tri-glial dysregulation and identify inflammatory microglia as a therapeutic target to abrogate chemotherapy-related cognitive impairment. VIDEO ABSTRACT.
Keywords: OPC; astrocyte; chemobrain; chemotherapy; chemotherapy-related cognitive impairment; microglia; myelin; oligodendrocyte.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests:
The authors declare no competing interests.
Figures





Comment in
-
A glial contribution to 'chemobrain'.Nat Rev Neurosci. 2019 Feb;20(2):67. doi: 10.1038/s41583-018-0115-z. Nat Rev Neurosci. 2019. PMID: 30602722 No abstract available.
-
Clearing the Fog surrounding Chemobrain.Cell. 2019 Jan 10;176(1-2):2-4. doi: 10.1016/j.cell.2018.12.027. Cell. 2019. PMID: 30633904
References
-
- Adamsky A,K. A; Kreisel T; Doron A; Ozeri-Engelhard N; Melcer T; Refaeli R; Horn H; Regev L; Groysman M; London M; Goshen I (2018). Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 1–13. - PubMed
-
- American Cancer Society (2016). Cancer Treatment & Survivorship Facts & Figures 2016–2017.
-
- Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, Last BF, Grootenhuis MA, and Schouten-van Meeteren AY (2009). White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys 74, 837–843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

