The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold
- PMID: 30529089
- PMCID: PMC6349489
- DOI: 10.1016/j.antiviral.2018.11.016
The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold
Abstract
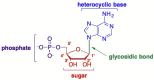
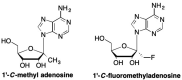
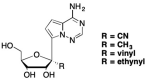
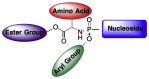
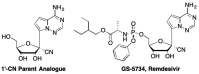
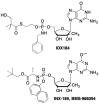
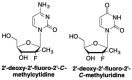
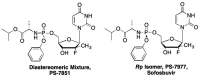
This is the second of two invited articles reviewing the development of nucleoside analogue antiviral drugs, written for a target audience of virologists and other non-chemists, as well as chemists who may not be familiar with the field. As with the first paper, rather than providing a chronological account, we have chosen to examine particular examples of structural modifications made to nucleoside analogues that have proven fruitful as various antiviral, anticancer, and other therapeutics. The first review covered the more common, and in most cases, single modifications to the sugar and base moieties of the nucleoside scaffold. This paper focuses on more recent developments, especially nucleoside analogues that contain more than one modification to the nucleoside scaffold. We hope that these two articles will provide an informative historical perspective of some of the successfully designed analogues, as well as many candidate compounds that encountered obstacles.
Keywords: Anti-cancer; Antiviral; Nucleoside analogues; Prodrugs; Structural modifications.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures






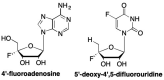

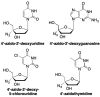


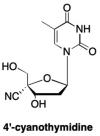
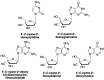

References
-
- Abele R., Alberto P., Kaplan S., Siegenthaler P., Hofmann V., Ryssel H.J., Hartmann D., Holdener E.E., Cavalli F. Phase II study of doxifluridine in advanced colorectal adenocarcinoma. J. Clin. Oncol. 1983;1:750–754. - PubMed
-
- Abele R., Kaplan E., Grossenbacher R., Schmid H.J., Cavalli F. Phase II study of doxifluridine in advanced squamous cell carcinoma of the head and neck. Eur. J. Cancer Clin. Oncol. 1984;20:333–336. - PubMed
-
- Ajmera S., Bapat A.R., Danenberg P.V., Stephanian E. Synthesis and interaction with uridine phosphorylase of 5'-deoxy-4',5-difluorouridine, a new prodrug of 5-fluorouracil. J. Med. Chem. 1988;31:1094–1098. - PubMed
-
- Ajmera S., Bapat A.R., Danenberg P.V., Stephanian E. Synthesis and interaction with uridine phosphorylase of 5'-deoxy-4',5-difluorouridine, a new prodrug of 5-fluorouracil. J. Med. Chem. 1988;31:1094–1098. - PubMed
-
- Alberto P., Jungi W.F., Siegenthaler P., Mermillod B., Obrecht J.P., Decoster G., Cavalli F. A phase II study of doxifluridine in patients with advanced breast cancer. Eur. J. Cancer. 1988;24:565–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

