Strategies for chemoenzymatic synthesis of carbohydrates
- PMID: 30529493
- PMCID: PMC6342554
- DOI: 10.1016/j.carres.2018.11.014
Strategies for chemoenzymatic synthesis of carbohydrates
Abstract
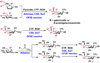
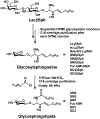
Carbohydrates are structurally complex but functionally important biomolecules. Therefore, they have been challenging but attractive synthetic targets. While substantial progress has been made on advancing chemical glycosylation methods, incorporating enzymes into carbohydrate synthetic schemes has become increasingly practical as more carbohydrate biosynthetic and metabolic enzymes as well as their mutants with synthetic application are identified and expressed for preparative and large-scale synthesis. Chemoenzymatic strategies that integrate the flexibility of chemical derivatization with enzyme-catalyzed reactions have been extremely powerful. Briefly summarized here are our experiences on developing one-pot multienzyme (OPME) systems and representative chemoenzymatic strategies from others using glycosyltransferase-catalyzed reactions for synthesizing diverse structures of oligosaccharides, polysaccharides, and glycoconjugates. These strategies allow the synthesis of complex carbohydrates including those containing naturally occurring carbohydrate postglycosylational modifications (PGMs) and non-natural functional groups. By combining these srategies with facile purification schemes, synthetic access to the diverse space of carbohydrate structures can be automated and will not be limited to specialists.
Keywords: Carbohydrate synthesis; Chemoenzymatic synthesis; Enzyme engineering; Glycolipid; Glycosyltransferase; Regioselective.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

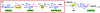

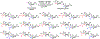
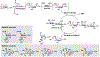
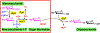




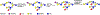

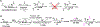
References
-
- Varki A, Kornfeld S, in: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (Eds.) Essentials of Glycobiology, Cold Spring Harbor (NY: ), 2015, pp. 1–18. - PubMed
-
- Hanson S, Best M, Bryan MC, Wong C-H, Chemoenzymatic synthesis of oligosaccharides and glycoproteins, Trends Biochem. Sci 29 (2004) 656–663. - PubMed
-
- Gloster TM, Development of inhibitors as research tools for carbohydrate-processing enzymes, Biochem. Soc. Trans 40 (2012) 913–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

