Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges
- PMID: 30529598
- PMCID: PMC6494979
- DOI: 10.1016/j.jtho.2018.11.023
Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges
Abstract
The present review is an update of the research and development efforts regarding the use of molecular biomarkers in the lung cancer screening setting. The two main unmet clinical needs, namely, the refinement of risk to improve the selection of individuals undergoing screening and the characterization of undetermined nodules found during the computed tomography-based screening process are the object of the biomarkers described in the present review. We first propose some principles to optimize lung cancer biomarker discovery projects. Then, we summarize the discovery and developmental status of currently promising molecular candidates, such as autoantibodies, complement fragments, microRNAs, circulating tumor DNA, DNA methylation, blood protein profiling, or RNA airway or nasal signatures. We also mention other emerging biomarkers or new technologies to follow, such as exhaled breath biomarkers, metabolomics, sputum cell imaging, genetic predisposition studies, and the integration of next-generation sequencing into study of circulating DNA. We also underline the importance of integrating different molecular technologies together with imaging, radiomics, and artificial intelligence. We list a number of completed, ongoing, or planned trials to show the clinical utility of molecular biomarkers. Finally, we comment on future research challenges in the field of biomarkers in the context of lung cancer screening and propose a design of a trial to test the clinical utility of one or several candidate biomarkers.
Keywords: Biomarkers; Clinical utility; Lung cancer; Screening; Test validation; Trial design.
Copyright © 2018 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest:
NP is advisor for and received honoraria from AZ, BI, BMS, Lilly, MSD, Novartis, Pfizer, Roche, NovellusDx, FMI, Gaurdant360 and is a co-inventor in breath analysis in lung cancer. DA, RP and LMM are listed as co-inventors of two patents related to the use of complement fragments as biomarkers. G.S and M.B. are co-inventors for three patent applications regarding the miRNA signature MSC. These patents were licensed to a private company, Gensignia Life Science, under regulations of Fondazione IRCCS Istituto Nazionale dei Tumori of Milan. JJZ is an employee and shareholder of VisionGate, Inc. AS is an employee of JNJ and prior consultant to Veracyte Inc. PJM has participated in the past 12 months in advisory board discussions for Exact Sciences, SEER, and Grail. LMS has received professional fees for speaking engagements regarding lung cancer screening and received an unrestricted grant from Menarini in support of the Fundación Jimenez Díaz’s lung cancer screening program.
Figures
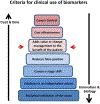


References
-
- Henschke CI, Shaham D, Yankelevitz DF, et al. CT screening for lung cancer: Significance of diagnoses in its baseline cycle. Clin Imaging. 2006;30(1):11–15. - PubMed
-
- Yousaf-Khan U, Van Der Aalst C, De Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: The effect of a 2.5-year screening interval. Thorax. 2017;72(1):48–56. - PubMed
-
- De Koning H, Van Der Aalst C, Ten Haaf MO K. Effects of volume CT lung cancer screening: mortality results of the NELSON randomized-controlled population trial. In: IASLC. ; 2018.
-
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–e766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

