A Novel Just-in-Time Contextual Mobile App Intervention to Reduce Sodium Intake in Hypertension: Protocol and Rationale for a Randomized Controlled Trial (LowSalt4Life Trial)
- PMID: 30530462
- PMCID: PMC6303672
- DOI: 10.2196/11282
A Novel Just-in-Time Contextual Mobile App Intervention to Reduce Sodium Intake in Hypertension: Protocol and Rationale for a Randomized Controlled Trial (LowSalt4Life Trial)
Abstract
Background: High sodium intake is a significant public health problem in the United States. Interventions that lower sodium intake can decrease blood pressure and improve cardiovascular outcomes. Restaurants and grocery stores are prime targets for intervention with about 77% of all sodium intake in the average US diet coming from processed and restaurant foods.
Objective: This study proposes that a mobile app intervention that promotes low-sodium alternatives at grocery stores and restaurants will reduce dietary intake of sodium and improve confidence following a low-sodium diet in hypertension.
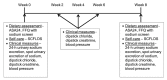
Methods: In this single-center, prospective, open-label study, patients will be randomized to a mobile app or usual care for 8 weeks. We will randomize 50 patients (age>18 years) diagnosed with hypertension and on antihypertensive therapy for at least 3 months in a 1:1 manner stratified by gender. Study subjects will receive the mobile app, LowSalt4Life, or usual dietary advice for 8 weeks. LowSalt4Life provides a multifaceted intervention based on just-in-time contextual tailored messages at grocery stores and restaurants. The primary endpoint is the change in the estimated 24-hour urinary excretion of sodium from spot urine. Secondary outcomes include change in the sodium content of the food frequency questionnaire, confidence in following a low-sodium diet, urine chloride and creatinine dipsticks, and blood pressure.
Results: The project was funded in May 2016 until April 2018. This trial is currently enrolling patients. To date, 26 of the 50 patients needed have been enrolled. Results will be available in the Spring of 2019.
Conclusions: This randomized controlled trial will test the efficacy of just-in-time contextual tailored messages through a novel mobile app 8-week intervention on urinary sodium excretion in patients with hypertension. We will address a critical evidence gap in the care of patients with hypertension. If effective, this intervention could be scaled to assess effects on blood pressure and cardiovascular events in hypertension.
Trial registration: ClinicalTrials.gov NCT03099343; https://clinicaltrials.gov/ct2/show/NCT03099343 (Archived by WebCite at http://www.webcitation.org/735HNzKlQ).
International registered report identifier (irrid): PRR1-10.2196/11282.
Keywords: geofencing; hypertension; mobile phone; sodium intake.
©Michael P Dorsch, Lawrence C An, Scott L Hummel. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 07.12.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
Effects of a Novel Contextual Just-In-Time Mobile App Intervention (LowSalt4Life) on Sodium Intake in Adults With Hypertension: Pilot Randomized Controlled Trial.JMIR Mhealth Uhealth. 2020 Aug 10;8(8):e16696. doi: 10.2196/16696. JMIR Mhealth Uhealth. 2020. PMID: 32663139 Free PMC article. Clinical Trial.
-
Examining the Individual Response to a Low-Sodium Diet in Patients with Hypertension: Protocol for a Pilot Randomized Controlled Trial.JMIR Res Protoc. 2023 Feb 13;12:e39058. doi: 10.2196/39058. JMIR Res Protoc. 2023. PMID: 36780210 Free PMC article.
-
Using the Social-Local-Mobile App for Smoking Cessation in the SmokeFreeBrain Project: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2018 Dec 6;7(12):e12464. doi: 10.2196/12464. JMIR Res Protoc. 2018. PMID: 30522992 Free PMC article.
-
Technologies for Innovative Monitoring to Reduce Blood Pressure and Change Lifestyle Using Mobile Phones in Adult and Elderly Populations (TIM Study): Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2018 Aug 7;7(8):e169. doi: 10.2196/resprot.9619. JMIR Res Protoc. 2018. PMID: 30087093 Free PMC article.
-
Effectiveness of a Sodium-Reduction Smartphone App and Reduced-Sodium Salt to Lower Sodium Intake in Adults With Hypertension: Findings From the Salt Alternatives Randomized Controlled Trial.JMIR Mhealth Uhealth. 2023 Mar 9;11:e43675. doi: 10.2196/43675. JMIR Mhealth Uhealth. 2023. PMID: 36892914 Free PMC article. Clinical Trial.
Cited by
-
Patient-centered mobile health technology intervention to improve self-care in patients with chronic heart failure: Protocol for a feasibility randomized controlled trial.Contemp Clin Trials. 2021 Jul;106:106433. doi: 10.1016/j.cct.2021.106433. Epub 2021 May 13. Contemp Clin Trials. 2021. PMID: 33991686 Free PMC article.
-
Effects of a Novel Contextual Just-In-Time Mobile App Intervention (LowSalt4Life) on Sodium Intake in Adults With Hypertension: Pilot Randomized Controlled Trial.JMIR Mhealth Uhealth. 2020 Aug 10;8(8):e16696. doi: 10.2196/16696. JMIR Mhealth Uhealth. 2020. PMID: 32663139 Free PMC article. Clinical Trial.
-
An Overview of Innovative Approaches to Support Timely and Agile Health Communication Research and Practice.Int J Environ Res Public Health. 2022 Nov 16;19(22):15073. doi: 10.3390/ijerph192215073. Int J Environ Res Public Health. 2022. PMID: 36429796 Free PMC article. Review.
-
Nitric Oxide Alleviated High Salt-Induced Cardiomyocyte Apoptosis and Autophagy Independent of Blood Pressure in Rats.Front Cell Dev Biol. 2021 Apr 29;9:646575. doi: 10.3389/fcell.2021.646575. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996809 Free PMC article.
-
Microrandomized Trial Design for Evaluating Just-in-Time Adaptive Interventions Through Mobile Health Technologies for Cardiovascular Disease.Circ Cardiovasc Qual Outcomes. 2021 Feb;14(2):e006760. doi: 10.1161/CIRCOUTCOMES.120.006760. Epub 2021 Jan 12. Circ Cardiovasc Qual Outcomes. 2021. PMID: 33430608 Free PMC article. Clinical Trial.
References
-
- Strazzullo P, D'Elia L, Kandala N, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009 Nov 24;339:b4567. http://europepmc.org/abstract/MED/19934192 - PMC - PubMed
-
- Dietary Guidelines Advisory Committee . U.S. Department of Agriculture and U.S. Department of Health and Human Services. Washington, DC: U.S. Government Printing Office; 2010. [2018-06-12]. Dietary Guidelines for Americans, 2010. 7th Edition https://health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf .
-
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH, DASH-Sodium CRG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. - DOI - PubMed
-
- He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014 Apr 14;4(4):e004549. doi: 10.1136/bmjopen-2013-004549. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=24732242 bmjopen-2013-004549 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

