Radiosensitivity Is an Acquired Vulnerability of PARPi-Resistant BRCA1-Deficient Tumors
- PMID: 30530501
- PMCID: PMC6366562
- DOI: 10.1158/0008-5472.CAN-18-2077
Radiosensitivity Is an Acquired Vulnerability of PARPi-Resistant BRCA1-Deficient Tumors
Abstract
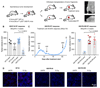
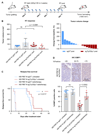
The defect in homologous recombination (HR) found in BRCA1-associated cancers can be therapeutically exploited by treatment with DNA-damaging agents and PARP inhibitors. We and others previously reported that BRCA1-deficient tumors are initially hypersensitive to the inhibition of topoisomerase I/II and PARP, but acquire drug resistance through restoration of HR activity by the loss of end-resection antagonists of the 53BP1/RIF1/REV7/Shieldin/CST pathway. Here, we identify radiotherapy as an acquired vulnerability of 53BP1;BRCA1-deficient cells in vitro and in vivo. In contrast to the radioresistance caused by HR restoration through BRCA1 reconstitution, HR restoration by 53BP1 pathway inactivation further increases radiosensitivity. This highlights the relevance of this pathway for the repair of radiotherapy-induced damage. Moreover, our data show that BRCA1-mutated tumors that acquire drug resistance due to BRCA1-independent HR restoration can be targeted by radiotherapy. SIGNIFICANCE: These findings uncover radiosensitivity as a novel, therapeutically viable vulnerability of BRCA1-deficient mouse mammary cells that have acquired drug resistance due to the loss of the 53BP1 pathway.
©2018 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed
Figures


References
-
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nature reviews Cancer. 2005;5:689–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

