A Novel, Multiple-Antigen Pneumococcal Vaccine Protects against Lethal Streptococcus pneumoniae Challenge
- PMID: 30530620
- PMCID: PMC6386546
- DOI: 10.1128/IAI.00846-18
A Novel, Multiple-Antigen Pneumococcal Vaccine Protects against Lethal Streptococcus pneumoniae Challenge
Erratum in
-
Correction for Chan et al., "A Novel, Multiple-Antigen Pneumococcal Vaccine Protects against Lethal Streptococcus pneumoniae Challenge".Infect Immun. 2022 Jan 25;90(1):e0063921. doi: 10.1128/IAI.00639-21. Epub 2022 Jan 25. Infect Immun. 2022. PMID: 35076290 Free PMC article. No abstract available.
Corrected and republished in
-
Corrected and Republished from: "A Novel, Multiple-Antigen Pneumococcal Vaccine Protects against Lethal Streptococcus pneumoniae Challenge".Infect Immun. 2022 Jan 25;90(1):e0084618a. doi: 10.1128/IAI.00846-18a. Epub 2018 Dec 10. Infect Immun. 2022. PMID: 35076289 Free PMC article.
Abstract
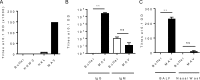
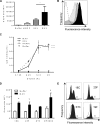
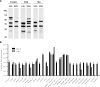
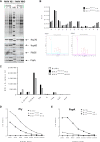
Current vaccination against Streptococcus pneumoniae uses vaccines based on capsular polysaccharides from selected serotypes and has led to nonvaccine serotype replacement disease. We have investigated an alternative serotype-independent approach, using multiple-antigen vaccines (MAV) prepared from S. pneumoniae TIGR4 lysates enriched for surface proteins by a chromatography step after culture under conditions that induce expression of heat shock proteins (Hsp; thought to be immune adjuvants). Proteomics and immunoblot analyses demonstrated that, compared to standard bacterial lysates, MAV was enriched with Hsps and contained several recognized protective protein antigens, including pneumococcal surface protein A (PspA) and pneumolysin (Ply). Vaccination of rodents with MAV induced robust antibody responses to multiple serotypes, including nonpneumococcal conjugate vaccine serotypes. Homologous and heterologous strains of S. pneumoniae were opsonized after incubation in sera from vaccinated rodents. In mouse models, active vaccination with MAV significantly protected against pneumonia, while passive transfer of rabbit serum from MAV-vaccinated rabbits significantly protected against sepsis caused by both homologous and heterologous S. pneumoniae strains. Direct comparison of MAV preparations made with or without the heat shock step showed no clear differences in protein antigen content and antigenicity, suggesting that the chromatography step rather than Hsp induction improved MAV antigenicity. Overall, these data suggest that the MAV approach may provide serotype-independent protection against S. pneumoniae.
Keywords: Streptococcus pneumoniae; multiple-antigen vaccine; protein antigen; vaccines.
Copyright © 2019 Chan et al.
Figures


References
-
- Sørensen UB, Henrichsen J, Chen HC, Szu SC, Sorensen UBS, Henrichsen J, Chen HC, Szu SC. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog 8:325–334. doi:10.1016/0882-4010(90)90091-4. - DOI - PubMed
-
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, Takei Y, Gabazza EC. 2010. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 340:c1004. doi:10.1136/bmj.c1004. - DOI - PMC - PubMed
-
- Webster J, Theodoratou E, Nair H, Seong AC, Zgaga L, Huda T, Johnson HL, Madhi S, Rubens C, Zhang JSF, El Arifeen S, Krause R, Jacobs TA, Brooks AW, Campbell H, Rudan I. 2011. An evaluation of emerging vaccines for childhood pneumococcal pneumonia. BMC Public Health 11:S26. doi:10.1186/1471-2458-11-S3-S26. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

