Fat-regulating phosphatidic acid phosphatase: a review of its roles and regulation in lipid homeostasis
- PMID: 30530634
- PMCID: PMC6314256
- DOI: 10.1194/jlr.S087452
Fat-regulating phosphatidic acid phosphatase: a review of its roles and regulation in lipid homeostasis
Abstract
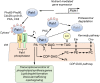
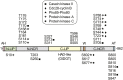
Phosphatidic acid (PA) phosphatase is an evolutionarily conserved enzyme that plays a major role in lipid homeostasis by controlling the cellular levels of its substrate, PA, and its product, diacylglycerol. These lipids are essential intermediates for the synthesis of triacylglycerol and membrane phospholipids; they also function in lipid signaling, vesicular trafficking, lipid droplet formation, and phospholipid synthesis gene expression. The importance of PA phosphatase to lipid homeostasis and cell physiology is exemplified in yeast, mice, and humans by a host of cellular defects and lipid-based diseases associated with loss or overexpression of the enzyme activity. In this review, we focus on the mode of action and regulation of PA phosphatase in the yeast Saccharomyces cerevisiae The enzyme Pah1 translocates from the cytosol to the nuclear/endoplasmic reticulum membrane through phosphorylation and dephosphorylation. Pah1 phosphorylation is mediated in the cytosol by multiple protein kinases, whereas dephosphorylation is catalyzed on the membrane surface by an integral membrane protein phosphatase. Posttranslational modifications of Pah1 also affect its catalytic activity and susceptibility to degradation by the proteasome. Additional mechanistic understanding of Pah1 regulation should be instrumental for the identification of small-molecule inhibitors or activators that can fine-tune PA phosphatase function and thereby restore lipid homeostasis.
Keywords: Nem1-Spo7 protein phosphatase complex; diacylglycerol; lipodystrophy; obesity; triacylglycerol.
Copyright © 2019 Carman and Han.
Conflict of interest statement
The authors declare that there are no conflicts of interest with the contents of this article.
Figures

References
-
- Smith S. W., Weiss S. B., and Kennedy E. P.. 1957. The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 228: 915–922. - PubMed
-
- Kates M. 1955. Hydrolysis of lecithin by plant plastid enzymes. Can. J. Biochem. 33: 575–589. - PubMed
-
- Lin Y-P., and Carman G. M.. 1989. Purification and characterization of phosphatidate phosphatase from Saccharomyces cerevisiae. J. Biol. Chem. 264: 8641–8645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

