MTSS1/Src family kinase dysregulation underlies multiple inherited ataxias
- PMID: 30530649
- PMCID: PMC6310854
- DOI: 10.1073/pnas.1816177115
MTSS1/Src family kinase dysregulation underlies multiple inherited ataxias
Abstract
The genetically heterogeneous spinocerebellar ataxias (SCAs) are caused by Purkinje neuron dysfunction and degeneration, but their underlying pathological mechanisms remain elusive. The Src family of nonreceptor tyrosine kinases (SFK) are essential for nervous system homeostasis and are increasingly implicated in degenerative disease. Here we reveal that the SFK suppressor Missing-in-metastasis (MTSS1) is an ataxia locus that links multiple SCAs. MTSS1 loss results in increased SFK activity, reduced Purkinje neuron arborization, and low basal firing rates, followed by cell death. Surprisingly, mouse models for SCA1, SCA2, and SCA5 show elevated SFK activity, with SCA1 and SCA2 displaying dramatically reduced MTSS1 protein levels through reduced gene expression and protein translation, respectively. Treatment of each SCA model with a clinically approved Src inhibitor corrects Purkinje neuron basal firing and delays ataxia progression in MTSS1 mutants. Our results identify a common SCA therapeutic target and demonstrate a key role for MTSS1/SFK in Purkinje neuron survival and ataxia progression.
Keywords: BAR domain proteins; Src kinase; actin cytoskeleton; neurodegeneration; spinocerebellar ataxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
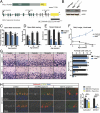

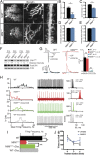

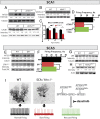
References
-
- Inoue T, et al. Calcium dynamics and electrophysiological properties of cerebellar Purkinje cells in SCA1 transgenic mice. J Neurophysiol. 2001;85:1750–1760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

