Improving vaccines against Streptococcus pneumoniae using synthetic glycans
- PMID: 30530654
- PMCID: PMC6310808
- DOI: 10.1073/pnas.1811862115
Improving vaccines against Streptococcus pneumoniae using synthetic glycans
Abstract
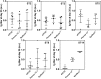
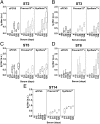
Streptococcus pneumoniae remains a deadly disease in small children and the elderly even though conjugate and polysaccharide vaccines based on isolated capsular polysaccharides (CPS) are successful. The most common serotypes that cause infection are used in vaccines around the world, but differences in geographic and demographic serotype distribution compromises protection by leading vaccines. The medicinal chemistry approach to glycoconjugate vaccine development has helped to improve the stability and immunogenicity of synthetic vaccine candidates for several serotypes leading to the induction of higher levels of specific protective antibodies. Here, we show that marketed CPS-based glycoconjugate vaccines can be improved by adding synthetic glycoconjugates representing serotypes that are not covered by existing vaccines. Combination (coformulation) of synthetic glycoconjugates with the licensed vaccines Prevnar13 (13-valent) and Synflorix (10-valent) yields improved 15- and 13-valent conjugate vaccines, respectively, in rabbits. A pentavalent semisynthetic glycoconjugate vaccine containing five serotype antigens (sPCV5) elicits antibodies with strong in vitro opsonophagocytic activity. This study illustrates that synthetic oligosaccharides can be used in coformulation with both isolated polysaccharide glycoconjugates to expand protection from existing vaccines and each other to produce precisely defined multivalent conjugated vaccines.
Keywords: Streptococcus pneumoniae; synthetic glycans; vaccine.
Conflict of interest statement
Conflict of interest statement: Glycoconjugates containing the synthetic glycan structures of all five Streptococcus pneumoniae serotypes (type 2, 3, 5, 8, and 14) capsular polysaccharide conjugate elicit opsonic antibodies and is included in patent “Pneumococcal oligosaccharide-protein conjugate composition” no. EP 16 179 133.0 filed by the inventors P.H.S., C.L.P., N.K., M.E., S.G.P., A.D.J.C., M.P.L., B.S., F.-F.X., P.K., and P.H.S. has a significant financial interest in “Vaxxilon,” a spinoff company that is developing vaccines based on synthetic oligosaccharide antigens.
Figures



References
-
- Centers for Disease Control and Prevention 2016 Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2016. Available at https://www.cdc.gov/abcs/reports-findings/survreports/spneu16.pdf. Accessed November 30, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

