SREBP-1a-stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1
- PMID: 30530672
- PMCID: PMC6310840
- DOI: 10.1073/pnas.1813458115
SREBP-1a-stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1
Abstract
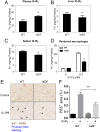
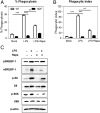
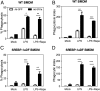
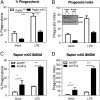
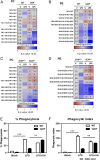
There is a growing appreciation for a fundamental connection between lipid metabolism and the immune response. Macrophage phagocytosis is a signature innate immune response to pathogen exposure, and cytoplasmic membrane expansion is required to engulf the phagocytic target. The sterol regulatory element binding proteins (SREBPs) are key transcriptional regulatory proteins that sense the intracellular lipid environment and modulate expression of key genes of fatty acid and cholesterol metabolism to maintain lipid homeostasis. In this study, we show that TLR4-dependent stimulation of macrophage phagocytosis requires mTORC1-directed SREBP-1a-dependent lipid synthesis. We also show that the phagocytic defect in macrophages from SREBP-1a-deficient mice results from decreased interaction between membrane lipid rafts and the actin cytoskeleton, presumably due to reduced accumulation of newly synthesized fatty acyl chains within major membrane phospholipids. We show that mTORC1-deficient macrophages also have a phagocytic block downstream from TLR4 signaling, and, interestingly, the reduced level of phagocytosis in both SREBP-1a- and mTORC1-deficient macrophages can be restored by ectopic SREBP-1a expression. Taken together, these observations indicate SREBP-1a is a major downstream effector of TLR4-mTORC1 directed interactions between membrane lipid rafts and the actin cytoskeleton that are required for pathogen-stimulated phagocytosis in macrophages.
Keywords: SREBPs; TLR4; lipid synthesis; mTORC1; macrophages.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Plüddemann A, Mukhopadhyay S, Gordon S. Innate immunity to intracellular pathogens: Macrophage receptors and responses to microbial entry. Immunol Rev. 2011;240:11–24. - PubMed
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Gagnon E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. - PubMed
-
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

