Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia
- PMID: 30530801
- PMCID: PMC6405333
- DOI: 10.1182/blood-2018-10-879429
Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia
Abstract
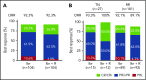
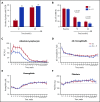
Ibrutinib, an oral covalent inhibitor of Bruton's tyrosine kinase, is an effective therapy for patients with chronic lymphocytic leukemia (CLL). To determine whether rituximab provides added benefit to ibrutinib, we conducted a randomized single-center trial of ibrutinib vs ibrutinib plus rituximab. Patients with CLL requiring therapy were randomized to receive 28-day cycles of once-daily ibrutinib 420 mg, either as a single agent (n = 104), or together with rituximab (375 mg/m2; n = 104), given weekly during cycle 1, then once per cycle until cycle 6. The primary end point was progression-free survival (PFS) in the intention-to-treat population. We enrolled 208 patients with CLL, 181 with relapsed CLL and 27 treatment-naive patients with high-risk disease (17p deletion or TP53 mutation). After a median follow-up of 36 months, the Kaplan-Meier estimates of PFS were 86% (95% confidence interval [CI], 76.6-91.9) for patients receiving ibrutinib, and 86.9% (95% CI, 77.3-92.6) for patients receiving ibrutinib plus rituximab. Similarly, response rates were the same in both arms (overall response rate, 92%). However, time to normalization of peripheral blood lymphocyte counts and time to complete remission were shorter, and residual disease levels in the bone marrow were lower, in patients receiving ibrutinib plus rituximab. We conclude that the addition of rituximab to ibrutinib in relapsed and treatment-naive high-risk patients with CLL failed to show improvement in PFS. However, patients treated with ibrutinib plus rituximab reached their remissions faster and achieved significantly lower residual disease levels. Given these results, ibrutinib as single-agent therapy remains current standard-of-care treatment in CLL. This trial was registered at www.clinicaltrials.gov as #NCT02007044.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.A.B., N.J., and S.O. received research funding from Pharmacyclics LLC. J.A.B. and N.J. are consultants for Janssen Pharmaceuticals, Inc. N.J. and P.T. have served as consultants for AbbVie and Pharmacyclics. J.L., M.C., and F.C. are employees of Pharmacyclics LLC. The remaining authors declare no competing interests.
Figures


Comment in
-
Targeting CD20 takes the backseat in CLL.Blood. 2019 Mar 7;133(10):1003-1004. doi: 10.1182/blood-2019-01-892695. Blood. 2019. PMID: 30846506 Free PMC article.
References
-
- Burger JA, O’Brien S. Evolution of CLL treatment—from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;15(8):510-527. - PubMed
-
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101-1110. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

