Contribution of non-circadian neurons to the temporal organization of locomotor activity
- PMID: 30530810
- PMCID: PMC6361196
- DOI: 10.1242/bio.039628
Contribution of non-circadian neurons to the temporal organization of locomotor activity
Abstract
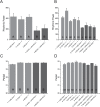
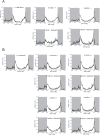
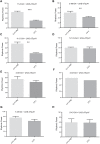
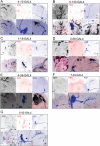
In the fruit fly, Drosophila melanogaster, the daily cycle of rest and activity is a rhythmic behavior that relies on the activity of a small number of neurons. The small ventral lateral neurons (sLNvs) are considered key in the control of locomotor rhythmicity. Previous work from our laboratory has showed that these neurons undergo structural remodeling on their axonal projections on a daily basis. Such remodeling endows sLNvs with the possibility to make synaptic contacts with different partners at different times throughout the day, as has been previously described. By using different genetic tools to alter membrane excitability of the sLNv putative postsynaptic partners, we tested their functional role in the control of locomotor activity. We also used optical imaging to test the functionality of these contacts. We found that these different neuronal groups affect the consolidation of rhythmic activity, suggesting that non-circadian cells are part of the circuit that controls locomotor activity. Our results suggest that new neuronal groups, in addition to the well-characterized clock neurons, contribute to the operations of the circadian network that controls locomotor activity in D. melanogaster.
Keywords: Connectivity; Drosophila; Locomotor rhythms; Non-circadian neurons; sLNvs.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Circadian pacemaker neurons change synaptic contacts across the day.Curr Biol. 2014 Sep 22;24(18):2161-2167. doi: 10.1016/j.cub.2014.07.063. Epub 2014 Aug 21. Curr Biol. 2014. PMID: 25155512 Free PMC article.
-
Autophagy as a new player in the regulation of clock neurons physiology of Drosophila melanogaster.Sci Rep. 2024 Mar 13;14(1):6085. doi: 10.1038/s41598-024-56649-3. Sci Rep. 2024. PMID: 38480808 Free PMC article.
-
Neuronal Activity in Non-LNv Clock Cells Is Required to Produce Free-Running Rest:Activity Rhythms in Drosophila.J Biol Rhythms. 2019 Jun;34(3):249-271. doi: 10.1177/0748730419841468. Epub 2019 Apr 17. J Biol Rhythms. 2019. PMID: 30994046 Free PMC article.
-
The circadian system: plasticity at many levels.Neuroscience. 2013 Sep 5;247:280-93. doi: 10.1016/j.neuroscience.2013.05.036. Epub 2013 May 29. Neuroscience. 2013. PMID: 23727010 Review.
-
Circadian Rhythms and Sleep in Drosophila melanogaster.Genetics. 2017 Apr;205(4):1373-1397. doi: 10.1534/genetics.115.185157. Genetics. 2017. PMID: 28360128 Free PMC article. Review.
Cited by
-
Regulation of Olfactory Associative Memory by the Circadian Clock Output Signal Pigment-Dispersing Factor (PDF).J Neurosci. 2020 Nov 18;40(47):9066-9077. doi: 10.1523/JNEUROSCI.0782-20.2020. Epub 2020 Oct 26. J Neurosci. 2020. PMID: 33106351 Free PMC article.
-
Synaptic Targets of Circadian Clock Neurons Influence Core Clock Parameters.bioRxiv [Preprint]. 2025 Feb 6:2025.01.30.635801. doi: 10.1101/2025.01.30.635801. bioRxiv. 2025. Update in: Sci Adv. 2025 Sep 5;11(36):eadw4666. doi: 10.1126/sciadv.adw4666. PMID: 39975067 Free PMC article. Updated. Preprint.
-
Nitric oxide mediates neuro-glial interaction that shapes Drosophila circadian behavior.PLoS Genet. 2020 Jun 29;16(6):e1008312. doi: 10.1371/journal.pgen.1008312. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32598344 Free PMC article.
-
Synaptic targets of circadian clock neurons influence core clock parameters.Sci Adv. 2025 Sep 5;11(36):eadw4666. doi: 10.1126/sciadv.adw4666. Epub 2025 Sep 3. Sci Adv. 2025. PMID: 40901945 Free PMC article.
-
Neurofibromin 1 in mushroom body neurons mediates circadian wake drive through activating cAMP-PKA signaling.Nat Commun. 2021 Oct 1;12(1):5758. doi: 10.1038/s41467-021-26031-2. Nat Commun. 2021. PMID: 34599173 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

