Efavirenz pharmacokinetics during pregnancy and infant washout
- PMID: 30530925
- PMCID: PMC6642905
- DOI: 10.3851/IMP3283
Efavirenz pharmacokinetics during pregnancy and infant washout
Abstract
Background: Limited data exist on efavirenz pharmacokinetics in HIV-positive pregnant women and neonatal washout.
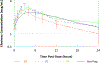
Methods: HIV-infected pregnant women receiving 600 mg efavirenz once daily had intensive steady-state 24-h pharmacokinetics profiles during the second trimester (2T), third trimester (3T) and 6-12 weeks postpartum (PP). Maternal and umbilical cord blood samples were drawn at delivery and neonatal washout pharmacokinetics were determined. Therapeutic targets were the estimated 10th percentile efavirenz area under the concentration-time curve (AUC) in non-pregnant historical controls (40.0 μg•h/ml) and a trough concentration (C24 h) of 1 μg/ml. Data were prospectively collected within two trials: IMPAACT P1026s (United States) and PANNA (Europe).
Results: Among 42 women studied, 15, 42 and 40 had efavirenz pharmacokinetic data available in 2T, 3T and PP, respectively. Median (range) 3T age 33 (20.7-43.5) years, weight 74 (50-132) kg and gestational age 33.4 (28.4-37.9 weeks). Efavirenz AUC during the 3T (60 μg•h/ml) was similar to that reported in non-pregnant adults (58 μg•h/ml). Exposure in the 2T was lower, but within the 0.80-1.25 range. C24 concentrations during pregnancy were lower compared to historical controls on 600 mg efavirenz, however, they were similar to the C24 concentrations after equally potent dose of 400 mg efavirenz. Cord blood/maternal plasma concentration ratio (range) was 0.67 (0.36-0.95). Among 23 infants with washout data available, median (interquartile range) elimination half-life was 65.6 h (40.6-129). HIV RNA viral loads at delivery were <400 and <50 copies/ml for 96.7% and 86.7% of women, respectively. In 3T and PP, respectively, 8/41 (19%) and 6/40 (15%) had AUC below target; 7/41 (17%) and 3/39 (8%) had C24 below target.
Conclusions: Efavirenz exposure was similar during pregnancy compared with PP, C24 was in line with C24 after 400 mg equipotent efavirenz dosing. Efavirenz readily crossed the placenta and infant elimination half-life was over twice that of maternal participants. Clincaltrials.gov identifiers: NCT00825929 and NCT00042289.
Conflict of interest statement
Disclosure statement
The authors report no conflicts of interest related to this work.
Figures


References
-
- DHHS.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2017; https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
-
- BHIVA. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015; http://www.bhiva.org/Guidelines.aspx. - PubMed
-
- WHO.Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection 2016; http://www.who.int/hiv/pub/arv/arv-2016/en/.
-
- de Ruiter A, Taylor GP, Clayden P, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012 (2014 interim review). HIV Med. 2014;15 Suppl 4:1–77. - PubMed
-
- De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med. 2002;162(3):355. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

