Five-Year Contraceptive Efficacy and Safety of a Levonorgestrel 52-mg Intrauterine System
- PMID: 30531565
- PMCID: PMC6319579
- DOI: 10.1097/AOG.0000000000003034
Five-Year Contraceptive Efficacy and Safety of a Levonorgestrel 52-mg Intrauterine System
Abstract
Objective: To assess the 5-year contraceptive efficacy and safety of a levonorgestrel (LNG) 52-mg intrauterine system (IUS) from an ongoing 10-year phase 3 contraceptive trial.
Methods: Study investigators enrolled 1,751 nulliparous and parous females aged 16-45 years and desiring contraception to receive a novel LNG 52-mg IUS at 29 centers in the United States, including reproductive health clinics, private offices, and university centers. Participants had scheduled follow-up visits four times during the first year. After year 1, study visits occurred every 6 months, with phone contact at the 3-month point between visits. We assessed the primary outcome of pregnancy rate (Pearl Index) in females aged 16-35 years at enrollment through 60 months. The safety evaluation included all females for their entire duration of participation.
Results: The 1,751 enrollees included 1,600 females aged 16-35 years and 151 aged 36-45 years. Successful IUS placement occurred in 1,714 (97.9%) participants. At the time of the data evaluation, 495 participants finished 5 years and 176 had entered the seventh year of IUS use. Nine pregnancies occurred, six of which were ectopic. The Pearl Indices for years 1 and 5 were 0.15 (95% CI 0.02-0.55) and 0.20 (95% CI 0.01-1.13) pregnancies per 100 women-years, respectively. The cumulative life-table pregnancy rate was 0.92% (0.46-1.82%) through 5 years. Participants aged 16-35 years at enrollment were significantly more likely to report new or worsening acne, dyspareunia, pelvic pain, and dysmenorrhea; participants aged 36-45 years at enrollment were more likely to report new or worsening weight increase. Discontinuation for adverse events occurred in 322 (18.8%) participants, most commonly related to expulsion (n=65 [3.8%]). Only 39 (2.2%) IUS users discontinued as a result of bleeding symptoms. Pelvic infection was diagnosed in 14 (0.8%) participants.
Conclusion: This LNG 52-mg IUS is highly effective and safe over 5 years of use in U.S. females.
Clinical trial registration: Clinicaltrials.gov, NCT00995150.
Funding source: Medicines360.
Figures
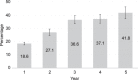
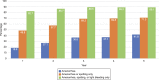
References
-
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62:1–60. - PubMed
-
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. - PubMed
-
- Lewis C, Darney P, Thiel de Bocanegra H. Intrauterine contraception: impact of provider training on participant knowledge and provision. Contraception 2013;88:226–31. - PubMed
-
- Ricketts S, Klingler G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspect Sex Reprod Health 2014;46:125–32. - PubMed

