Efficacy of Misoprostol Alone for First-Trimester Medical Abortion: A Systematic Review
- PMID: 30531568
- PMCID: PMC6309472
- DOI: 10.1097/AOG.0000000000003017
Efficacy of Misoprostol Alone for First-Trimester Medical Abortion: A Systematic Review
Abstract
Objective: To summarize available data on the effectiveness and safety of single-agent misoprostol for medical abortion in the first trimester.
Data sources: We searched MEDLINE, CABI, Cochrane, EMBASE, LILACS, the Web of Science, and ClinicalTrials.gov for English-language studies that evaluated misoprostol alone for abortion of a viable pregnancy in the first trimester.
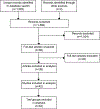
Methods of study selection: Our search yielded 1,562 citations, of which 38 included data from 53 trial groups that met our inclusion and exclusion criteria.
Tabulation, integration, and results: We abstracted data about each trial group, including study characteristics, treatment regimen, clinical protocol, number of women treated and followed, and numbers with outcomes of interest. We used meta-analytic methods and logistic regression to examine factors associated with surgical intervention after treatment. Among all 12,829 evaluable women, 2,536 (meta-analytic estimate 22.0%, 95% CI 18.8-25.5%) had surgical uterine evacuation. Multiple factors were significantly associated with this proportion, including misoprostol amount per dose and route of administration, loss to follow-up rate, publication date, geographic region, number of misoprostol doses, duration of dosing, and time between dosing and evaluation. Of 6,359 evaluable women, 384 (meta-analytic estimate 6.8%, 95% CI 5.3-8.5%) had ongoing pregnancies. At most 26 of 12,184 evaluable women (meta-analytic estimate 0.7%, 95% CI 0.4-1.0%) were transfused or hospitalized for abortion-related reasons. In trials that provided satisfaction data, most women were satisfied or very satisfied with the treatment (meta-analytic estimate 78%, 95% CI 71-85%).
Conclusions: Misoprostol alone is effective and safe and is a reasonable option for women seeking abortion in the first trimester. Research is indicated to further refine the regimen and to establish efficacy in the late first trimester.
Systematic review registration: PROSPERO, CRD42018083589.
Conflict of interest statement
Financial Disclosure
Mark A. Weaver has a consulting agreement with Gynuity Health Projects. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Figures
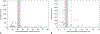
References
-
- Raymond EG, Shannon C, Weaver MA, Winikoff B. First-trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception 2013;87:26–37. - PubMed
-
- Chen MJ, Creinin MD. Mifepristone With Buccal Misoprostol for Medical Abortion: A Systematic Review. Obstet Gynecol 2015;126:12–21. - PubMed
-
- Abbas D, Chong E, Raymond EG. Outpatient medical abortion is safe and effective through 70 days gestation. Contraception 2015;92:197–9. - PubMed
-
- Raymond EG, Blanchard K, Blumenthal PD, et al. Sixteen Years of Overregulation: Time to Unburden Mifeprex. N Engl J Med 2017;376:790–4. - PubMed
-
- Moreno-Ruiz NL, Borgatta L, Yanow S, Kapp N, Wiebe ER, Winikoff B. Alternatives to mifepristone for early medical abortion. Int J Gynaecol Obstet 2007;96:212–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

