Efficient oxygen evolution electrocatalysis in acid by a perovskite with face-sharing IrO6 octahedral dimers
- PMID: 30531797
- PMCID: PMC6286314
- DOI: 10.1038/s41467-018-07678-w
Efficient oxygen evolution electrocatalysis in acid by a perovskite with face-sharing IrO6 octahedral dimers
Abstract
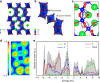
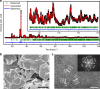
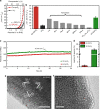
The widespread use of proton exchange membrane water electrolysis requires the development of more efficient electrocatalysts containing reduced amounts of expensive iridium for the oxygen evolution reaction (OER). Here we present the identification of 6H-phase SrIrO3 perovskite (6H-SrIrO3) as a highly active electrocatalyst with good structural and catalytic stability for OER in acid. 6H-SrIrO3 contains 27.1 wt% less iridium than IrO2, but its iridium mass activity is about 7 times higher than IrO2, a benchmark electrocatalyst for the acidic OER. 6H-SrIrO3 is the most active catalytic material for OER among the iridium-based oxides reported recently, based on its highest iridium mass activity. Theoretical calculations indicate that the existence of face-sharing octahedral dimers is mainly responsible for the superior activity of 6H-SrIrO3 thanks to the weakened surface Ir-O binding that facilitates the potential-determining step involved in the OER (i.e., O* + H2O → HOO* + H+ + e¯).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Pi Y, Shao Q, Wang P, Guo J, Huang X. General formation of monodisperse IrM (M=Ni, Co, Fe) bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting. Adv. Funct. Mater. 2017;27:1700886. doi: 10.1002/adfm.201700886. - DOI
Publication types
LinkOut - more resources
Full Text Sources

