Macromolecular-clustered facial amphiphilic antimicrobials
- PMID: 30531920
- PMCID: PMC6286373
- DOI: 10.1038/s41467-018-07651-7
Macromolecular-clustered facial amphiphilic antimicrobials
Abstract
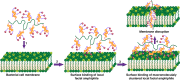
Bacterial infections and antibiotic resistance, particularly by Gram-negative pathogens, have become a global healthcare crisis. We report the design of a class of cationic antimicrobial polymers that cluster local facial amphiphilicity from repeating units to enhance interactions with bacterial membranes without requiring a globally conformational arrangement associated with highly unfavorable entropic loss. This concept of macromolecular architectures is demonstrated with a series of multicyclic natural product-based cationic polymers. We have shown that cholic acid derivatives with three charged head groups are more potent and selective than lithocholic and deoxycholic counterparts, particularly against Gram-negative bacteria. This is ascribed to the formation of true facial amphiphilicity with hydrophilic ion groups oriented on one face and hydrophobic multicyclic hydrocarbon structures on the opposite face. Such local facial amphiphilicity is clustered via a flexible macromolecular backbone in a concerted way when in contact with bacterial membranes.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, Atlanta, GA. CDC, https://www.cdc.gov/drugresistance/threat-report-2013 (2013).
-
- Lam, S. J. et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol.1, 16162 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

