Molecular Russian dolls
- PMID: 30531970
- PMCID: PMC6288134
- DOI: 10.1038/s41467-018-07673-1
Molecular Russian dolls
Abstract
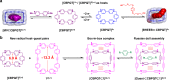
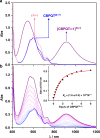
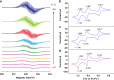
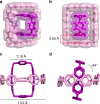
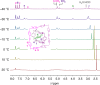
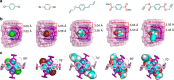
The host-guest recognition between two macrocycles to form hierarchical non-intertwined ring-in-ring assemblies remains an interesting and challenging target in noncovalent synthesis. Herein, we report the design and characterization of a box-in-box assembly on the basis of host-guest radical-pairing interactions between two rigid diradical dicationic cyclophanes. One striking feature of the box-in-box complex is its ability to host various 1,4-disubstituted benzene derivatives inside as a third component in the cavity of the smaller of the two diradical dicationic cyclophanes to produce hierarchical Russian doll like assemblies. These results highlight the utility of matching the dimensions of two different cyclophanes as an efficient approach for developing new hybrid supramolecular assemblies with radical-paired ring-in-ring complexes and smaller neutral guest molecules.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Pedersen CJ. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967;89:7017–7036. doi: 10.1021/ja01002a035. - DOI
-
- Pedersen CJ. Crystalline salt complexes of macrocyclic polyethers. J. Am. Chem. Soc. 1970;92:386–391. doi: 10.1021/ja00705a605. - DOI
-
- Dietrich B, Lehn JM, Sauvage. JP. Diaza-polyoxa-macrocycles et macrobicycles. Tetrahedron Lett. 1969;10:2885–2888. doi: 10.1016/S0040-4039(01)88299-X. - DOI
-
- Cram DJ, et al. Spherands—ligands whose binding of cations relieves enforced electron-electron repulsions. J. Am. Chem. Soc. 1979;101:6752–6754. doi: 10.1021/ja00516a048. - DOI
-
- Lehn JM. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 1988;27:89–112. doi: 10.1002/anie.198800891. - DOI
Publication types
LinkOut - more resources
Full Text Sources

