Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responses
- PMID: 30532004
- PMCID: PMC6784910
- DOI: 10.1038/s41386-018-0274-7
Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responses
Abstract
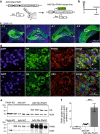
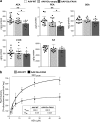
Endocannabinoid signaling via anandamide (AEA) is implicated in a variety of neuronal functions and considered a promising therapeutic target for numerous emotion-related disorders. The major AEA degrading enzyme is fatty acid amide hydrolase (FAAH). Genetic deletion and pharmacological inhibition of FAAH reduce anxiety and improve emotional responses and memory in rodents and humans. Complementarily, the mechanisms and impact of decreased AEA signaling remain to be delineated in detail. In the present study, using the Cre/loxP system combined with an adeno-associated virus (AAV)-mediated delivery system, FAAH was selectively overexpressed in hippocampal CA1-CA3 glutamatergic neurons of adult mice. This approach led to specific FAAH overexpression at the postsynaptic site of CA1-CA3 neurons, to increased FAAH enzymatic activity, and, in consequence, to decreased hippocampal levels of AEA and palmitoylethanolamide (PEA), but the levels of the second major endocannabinoid 2-arachidonoyl glycerol (2-AG) and of oleoylethanolamide (OEA) were unchanged. Electrophysiological recordings revealed an enhancement of both excitatory and inhibitory synaptic activity and of long-term potentiation (LTP). In contrast, excitatory and inhibitory long-term depression (LTD) and short-term synaptic plasticity, apparent as depolarization-induced suppression of excitation (DSE) and inhibition (DSI), remained unaltered. These changes in hippocampal synaptic activity were associated with an increase in anxiety-like behavior, and a deficit in object recognition memory and in extinction of aversive memory. This study indicates that AEA is not involved in hippocampal short-term plasticity, or eLTD and iLTD, but modulates glutamatergic transmission most likely via presynaptic sites, and that disturbances in this process impair learning and emotional responses.
Figures



Comment in
-
Buzzkill: the consequences of depleting anandamide in the hippocampus.Neuropsychopharmacology. 2019 Jul;44(8):1347-1348. doi: 10.1038/s41386-019-0357-0. Epub 2019 Mar 1. Neuropsychopharmacology. 2019. PMID: 30824852 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

