Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
- PMID: 30532014
- PMCID: PMC6347513
- DOI: 10.1038/s41557-018-0188-3
Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
Abstract
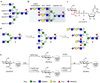
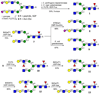
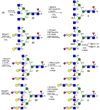
Contemporary chemoenzymatic approaches can provide highly complex multi-antennary N-linked glycans. These procedures are, however, very demanding and typically involve as many as 100 chemical steps to prepare advanced intermediates that can be diversified by glycosyltransferases in a branch-selective manner to give asymmetrical structures commonly found in nature. Only highly specialized laboratories can perform such syntheses, which greatly hampers progress in glycoscience. Here we describe a biomimetic approach in which a readily available bi-antennary glycopeptide can be converted in ten or fewer chemical and enzymatic steps into multi-antennary N-glycans that at each arm can be uniquely extended by glycosyltransferases to give access to highly complex asymmetrically branched N-glycans. A key feature of our approach is the installation of additional branching points using recombinant MGAT4 and MGAT5 in combination with unnatural sugar donors. At an appropriate point in the enzymatic synthesis, the unnatural monosaccharides can be converted into their natural counterpart, allowing each arm to be elaborated into a unique appendage.
Figures

Similar articles
-
Chemoenzymatic Synthesis of Tri-antennary N-Glycans Terminating in Sialyl-Lewisx Reveals the Importance of Glycan Complexity for Influenza A Virus Receptor Binding.Chemistry. 2024 Jun 6;30(32):e202401108. doi: 10.1002/chem.202401108. Epub 2024 May 8. Chemistry. 2024. PMID: 38567703 Free PMC article.
-
Chemoenzymatic Approach for the Preparation of Asymmetric Bi-, Tri-, and Tetra-Antennary N-Glycans from a Common Precursor.J Am Chem Soc. 2017 Jan 18;139(2):1011-1018. doi: 10.1021/jacs.6b12080. Epub 2017 Jan 6. J Am Chem Soc. 2017. PMID: 28002670 Free PMC article.
-
Substrate Preference and Interplay of Fucosyltransferase 8 and N-Acetylglucosaminyltransferases.J Am Chem Soc. 2017 Jul 19;139(28):9431-9434. doi: 10.1021/jacs.7b03729. Epub 2017 Jul 11. J Am Chem Soc. 2017. PMID: 28678517
-
Glycopeptide and glycoprotein synthesis involving unprotected carbohydrate building blocks.Med Res Rev. 2005 Nov;25(6):655-78. doi: 10.1002/med.20033. Med Res Rev. 2005. PMID: 15895471 Review.
-
Recent progress in chemoenzymatic synthesis of human glycans.Org Biomol Chem. 2024 Oct 2;22(38):7767-7785. doi: 10.1039/d4ob01006j. Org Biomol Chem. 2024. PMID: 39246045 Review.
Cited by
-
VaporSPOT: Parallel Synthesis of Oligosaccharides on Membranes.J Am Chem Soc. 2022 Nov 2;144(43):19832-19837. doi: 10.1021/jacs.2c07285. Epub 2022 Oct 21. J Am Chem Soc. 2022. PMID: 36269942 Free PMC article.
-
Sequential Glycosylation of Proteins with Substrate-Specific N-Glycosyltransferases.ACS Cent Sci. 2020 Feb 26;6(2):144-154. doi: 10.1021/acscentsci.9b00021. Epub 2020 Feb 19. ACS Cent Sci. 2020. PMID: 32123732 Free PMC article.
-
Recent Progress in Chemo-Enzymatic Methods for the Synthesis of N-Glycans.Front Chem. 2020 Jun 16;8:513. doi: 10.3389/fchem.2020.00513. eCollection 2020. Front Chem. 2020. PMID: 32612979 Free PMC article. Review.
-
Synthesis of glycopeptides and glycopeptide conjugates.Org Biomol Chem. 2022 Aug 24;20(33):6487-6507. doi: 10.1039/d2ob00829g. Org Biomol Chem. 2022. PMID: 35903971 Free PMC article. Review.
-
Chemoenzymatic Synthesis of Asymmetrically Branched Human Milk Oligosaccharide Lacto-N-Hexaose.Front Chem. 2022 May 31;10:905105. doi: 10.3389/fchem.2022.905105. eCollection 2022. Front Chem. 2022. PMID: 35711960 Free PMC article.
References
-
- Ohtsubo K & Marth JD Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 (2006). - PubMed
-
- Lauc G, Pezer M, Rudan I & Campbell H Mechanisms of disease: The human N-glycome. Biochim. Biophys. Acta 1860, 1574–1582 (2016). - PubMed
-
- Kiessling LL & Splain RA Chemical approaches to glycobiology. Annu. Rev. Biochem 79, 619–653 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

