Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
- PMID: 30532014
- PMCID: PMC6347513
- DOI: 10.1038/s41557-018-0188-3
Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
Abstract
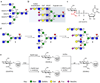
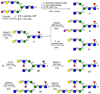
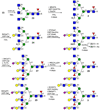
Contemporary chemoenzymatic approaches can provide highly complex multi-antennary N-linked glycans. These procedures are, however, very demanding and typically involve as many as 100 chemical steps to prepare advanced intermediates that can be diversified by glycosyltransferases in a branch-selective manner to give asymmetrical structures commonly found in nature. Only highly specialized laboratories can perform such syntheses, which greatly hampers progress in glycoscience. Here we describe a biomimetic approach in which a readily available bi-antennary glycopeptide can be converted in ten or fewer chemical and enzymatic steps into multi-antennary N-glycans that at each arm can be uniquely extended by glycosyltransferases to give access to highly complex asymmetrically branched N-glycans. A key feature of our approach is the installation of additional branching points using recombinant MGAT4 and MGAT5 in combination with unnatural sugar donors. At an appropriate point in the enzymatic synthesis, the unnatural monosaccharides can be converted into their natural counterpart, allowing each arm to be elaborated into a unique appendage.
Figures

References
-
- Ohtsubo K & Marth JD Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 (2006). - PubMed
-
- Lauc G, Pezer M, Rudan I & Campbell H Mechanisms of disease: The human N-glycome. Biochim. Biophys. Acta 1860, 1574–1582 (2016). - PubMed
-
- Kiessling LL & Splain RA Chemical approaches to glycobiology. Annu. Rev. Biochem 79, 619–653 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

