Novel broad spectrum virucidal molecules against enveloped viruses
- PMID: 30532192
- PMCID: PMC6285983
- DOI: 10.1371/journal.pone.0208333
Novel broad spectrum virucidal molecules against enveloped viruses
Abstract
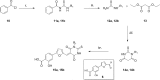
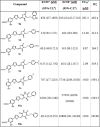
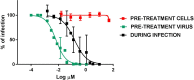
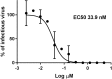
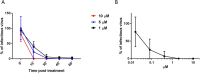
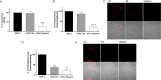
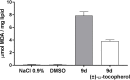
Viral infections are an important cause of death worldwide. Unfortunately, there is still a lack of antiviral drugs or vaccines for a large number of viruses, and this represents a remarkable challenge particularly for emerging and re-emerging viruses. For this reason, the identification of broad spectrum antiviral compounds provides a valuable opportunity for developing efficient antiviral therapies. Here we report on a class of rhodanine and thiobarbituric derivatives displaying a broad spectrum antiviral activity against seven different enveloped viruses including an HSV-2 acyclovir resistant strain with favorable selectivity indexes. Due to their selective action on enveloped viruses and to their lipid oxidation ability, we hypothesize a mechanism on the viral envelope that affects the fluidity of the lipid bilayer, thus compromising the efficiency of virus-cell fusion and preventing viral entry.
Conflict of interest statement
The commercial affiliation to Lead Discovery Siena srl does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- The top 10 causes of death [Internet]. World Health Organization. Available from: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
-
- Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319: 1802–1813. 10.1001/jama.2018.3795 - DOI - PubMed
-
- Dengue and severe dengue [Internet]. World Health Organization. Available from: http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
-
- Wisskirchen K, Lucifora J, Michler T, Protzer U. New pharmacological strategies to fight enveloped viruses. Trends Pharmacol Sci. 2014;35: 470–478. 10.1016/j.tips.2014.06.004 - DOI - PMC - PubMed
-
- Mackenzie JS, Jeggo M. Reservoirs and vectors of emerging viruses. Curr Opin Virol. 2013;3: 170–179. 10.1016/j.coviro.2013.02.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

