Securidaca-saponins are natural inhibitors of AKT, MCL-1, and BCL2L1 in cervical cancer cells
- PMID: 30532593
- PMCID: PMC6245348
- DOI: 10.2147/CMAR.S163328
Securidaca-saponins are natural inhibitors of AKT, MCL-1, and BCL2L1 in cervical cancer cells
Erratum in
-
Erratum: Securidaca-saponins are natural inhibitors of AKT, MCL-1, and BCL2L1 in cervical cancer cells [Corrigendum].Cancer Manag Res. 2019 Jan 15;11:691. doi: 10.2147/CMAR.S198251. eCollection 2019. Cancer Manag Res. 2019. PMID: 30697065 Free PMC article.
Abstract
Introduction: Scientific research is beginning to prove the connection between claims by African traditional medicine and the natural chemical specifics contained in medicinal plant Securidaca longipedunculata. Our previous studies showed that two natural saponin fractions (4A3 and 4A4) identified in the plant as triterpenoid glycosides are capable of activating apoptosis on cervical tumor cell lines. Considering this and some critical roles of human papillomavirus (HPV) E6 oncogene on cervical cells, by promoting carcinogenesis and cell survival, it became necessary to investigate the possible pathways for apoptosis transmission.
Methods: Tests conducted on relevant cervical tumor cell lines such as Caski and Bu25TK included the following: MTT assay; scratch assay (to determine cell migration/invasion); fluorescence microscopy with Annexin V-fluorescein isothiocyanate, muscle progenitor cell) and propidium iodide staining; and finally reverse transcriptase quantitative PCR (RT-qPCR) for gene analysis.
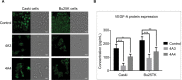
Results: Reduced cell proliferation was observed due to activities of 4A3 and 4A4 fractions, with half-maximal inhibitory concentration (IC50) of 7.03 and 16.39 μg/mL, respectively, on Caski cell line. A significant reduction in cell migration occurred within 48 and 72 hours, respectively, for Caski and Bu25TK cell lines. Late apoptosis was activated by 4A3, staining both Annexin V and PI, in contrast to 4A4's early apoptosis. RT-qPCR data revealed a fold change (FC) inhibition of antiapoptotic proteins such as MCL-1 and BCL2L1, with diminished level of AKT-3, VEGFA, MALAT1, etc. The expression of p53, proapoptotic BAD, and caspase-8 was nonsignificant.
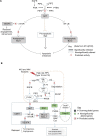
Conclusion: The low expression of AKT-3 and antiapoptotic proteins (MCL-1 and BCL2L1), as well as VEGFA, could simply be an indication for possible suppression of cell survival mechanisms via multiple channels. We therefore conclude that 4A3 and 4A4 fractions mediate activity via the inhibition of phosphatidylinositol-3-OH kinase (PI3K)-AKT/mTOR/NF-kB-dependent antiapoptotic stimuli. Further studies are ongoing to reveal the chemical structures and compositions of these two fractions.
Keywords: AKT-3; MCL-1 and BCL2L1 inhibition; RT-qPCR gene analysis; early apoptosis; triterpenoid saponins.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Ferulic Acid Induces Apoptosis of HeLa and Caski Cervical Carcinoma Cells by Down-Regulating the Phosphatidylinositol 3-Kinase (PI3K)/Akt Signaling Pathway.Med Sci Monit. 2020 Jan 27;26:e920095. doi: 10.12659/MSM.920095. Med Sci Monit. 2020. PMID: 31983729 Free PMC article.
-
Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism.Cancer Res. 2013 Feb 15;73(4):1340-51. doi: 10.1158/0008-5472.CAN-12-1365. Epub 2012 Dec 12. Cancer Res. 2013. PMID: 23243017 Free PMC article.
-
Anti-proliferative effect of RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by inhibiting the PI3K/Akt/mTOR signaling pathway and NF-κB activation.Naunyn Schmiedebergs Arch Pharmacol. 2016 Jun;389(6):573-84. doi: 10.1007/s00210-016-1217-7. Epub 2016 Mar 3. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 26935715
-
Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway.Eur Rev Med Pharmacol Sci. 2018 Dec;22(23):8135-8144. doi: 10.26355/eurrev_201812_16505. Eur Rev Med Pharmacol Sci. 2018. PMID: 30556851
-
[Inhibition effect of active fraction from clove on PI3K/Akt/mTOR signaling pathway to induce apoptosis of human colon cancer HCT116 cells].Zhongguo Zhong Yao Za Zhi. 2021 Mar;46(5):1197-1204. doi: 10.19540/j.cnki.cjcmm.20201027.401. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 33787115 Chinese.
Cited by
-
Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents.Front Endocrinol (Lausanne). 2021 Jan 21;11:612396. doi: 10.3389/fendo.2020.612396. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33552000 Free PMC article. Review.
-
Expression and gene regulation network of TYMS and BCL2L1 in colorectal cancer based on data mining.PeerJ. 2021 Jun 2;9:e11368. doi: 10.7717/peerj.11368. eCollection 2021. PeerJ. 2021. PMID: 34141464 Free PMC article.
-
Identification of Core Genes Involved in the Progression of Cervical Cancer Using an Integrative mRNA Analysis.Int J Mol Sci. 2020 Oct 3;21(19):7323. doi: 10.3390/ijms21197323. Int J Mol Sci. 2020. PMID: 33023042 Free PMC article.
-
Human papillomavirus associated cervical lesion: pathogenesis and therapeutic interventions.MedComm (2020). 2023 Sep 14;4(5):e368. doi: 10.1002/mco2.368. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37719443 Free PMC article. Review.
-
Circular RNA CDR1as Exerts Oncogenic Properties Partially through Regulating MicroRNA 641 in Cholangiocarcinoma.Mol Cell Biol. 2020 Jul 14;40(15):e00042-20. doi: 10.1128/MCB.00042-20. Print 2020 Jul 14. Mol Cell Biol. 2020. PMID: 32423991 Free PMC article.
References
-
- Burz C, Berindan-Neagoe I, Balacescu O, Irimie A. Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48(6):811–821. - PubMed
-
- Berindan-Neagoe I, Braicu C, Tudoran O, Balacescu O, Irimie A. Early apoptosis signals induced by a low dose of epigallocatechin 3-gallate interfere with apoptotic and cell death pathways. J Nanosci Nanotechnol. 2012;12(3):2113–2119. - PubMed
-
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528–537. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

