Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate
- PMID: 30532686
- PMCID: PMC6282430
- DOI: 10.1080/16512235.2018.1549922
Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate
Abstract
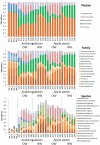
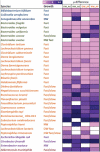
Background: Nutrient and energy metabolism in human colon depends on bacterial growth rate that is determined by the colonic transit rate. Objective: A novel approach, De-stat culture was used to distinguish the fast and slow growing sub-populations from fecal microbiota. Design: The enrichment and metabolism of bacteria from pooled fecal cultures of children was studied at dilution rates D = 0.2-0.0 1/h in mucin-supplemented media containing either arabinogalactan or apple pectin. Results: The study revealed clear differentiation of the fecal microbiota at higher (above 0.1 1/h) and lower (below 0.1 1/h) dilution rates, along with metabolic changes. Similarity of the fast and slow growing bacteria was observed in two different fecal pools and on both substrates, suggesting the dilution rate as the main triggering parameter for selection of bacteria. At high dilution rates, the species Collinsella aerofaciens, Dorea longicatena, Escherichia coli, Lachnoclostridium torques, and different Bacteroides (B. caccae, B. fragilis, B. ovatus, B. thetaiotaomicron, B. vulgatus) were dominant in both media variants. At low dilution rates, Akkermansia muciniphila, Eisenbergiella tayi, Negativicoccus succinivorans, and a group of Ruminococcaceae became dominant in both media and in both fecal pools. This change in bacterial population accompanied by the increased production of propionic and butyric acids as well as higher consumption of alanine and branched chain amino acids at low dilution rates. Conclusions: The study suggests that specific growth rate has important effect on the dynamics of colon microbiota. Manipulation of the proportions of fast and slow growing gut bacteria through modulation of the transit rate could be a target in human nutrition studies. The De-stat study would enable to predict changes in microbiota composition associated with the decrease or increase of the colonic transit rate.
Keywords: Dietary fiber; apple pectin; arabinogalactan; colon; gut transit rate; mucin.
Figures



References
-
- Venema K, Carmo A.. Probiotics and prebiotics: current status and future trends In: Venema K, Carmo AP, editors. Probiotics and prebiotics: current research and future trends. Caister Academic Press; 2015. p. 3–12. DOI: 10.21775/9781910190098.01. - DOI
LinkOut - more resources
Full Text Sources
