Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection
- PMID: 30532738
- PMCID: PMC6265504
- DOI: 10.3389/fendo.2018.00702
Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection
Abstract
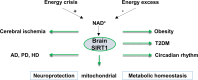
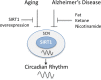
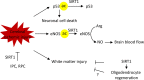
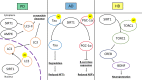
Sirtuins are evolutionarily conserved proteins that use nicotinamide adenine dinucleotide (NAD+) as a co-substrate in their enzymatic reactions. There are seven proteins (SIRT1-7) in the human sirtuin family, among which SIRT1 is the most conserved and characterized. SIRT1 in the brain, in particular, within the hypothalamus, plays crucial roles in regulating systemic energy homeostasis and circadian rhythm. Apart from this, SIRT1 has also been found to mediate beneficial effects in neurological diseases. In this review, we will first summarize how SIRT1 in the brain relates to obesity, type 2 diabetes, and circadian synchronization, and then we discuss the neuroprotective roles of brain SIRT1 in the context of cerebral ischemia and neurodegenerative disorders.
Keywords: Alzheimer's disease; Parkinson's disease; Sirt1; cerebral ischemia; circadian rhythms; obesity; type 2 diabetes mellitus.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

