Prevention of Elevation in Plasma Triacylglycerol with High-Dose Bezafibrate Treatment Abolishes Insulin Resistance and Attenuates Glucose Intolerance Induced by Short-Term Treatment with Dexamethasone in Rats
- PMID: 30532777
- PMCID: PMC6250034
- DOI: 10.1155/2018/3257812
Prevention of Elevation in Plasma Triacylglycerol with High-Dose Bezafibrate Treatment Abolishes Insulin Resistance and Attenuates Glucose Intolerance Induced by Short-Term Treatment with Dexamethasone in Rats
Abstract
Objective: Fibrates are used as lipid-lowering drugs and are well tolerated as cotreatments when glucose metabolism disturbances are also present. Synthetic glucocorticoids (GCs) are diabetogenic drugs that cause dyslipidemia, dysglycemia, glucose intolerance, and insulin resistance when in excess. Thus, we aimed to describe the potential of bezafibrate in preventing or attenuating the adverse effects of GCs on glucose and lipid homeostasis.
Methods: Male Wistar rats were treated with high-dose bezafibrate (300 mg/kg, body mass (b.m.)) daily for 28 consecutive days. In the last five days, the rats were also treated with dexamethasone (1 mg/kg, b.m.).
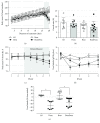
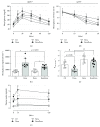
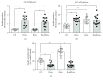
Results: Dexamethasone treatment reduced the body mass gain and food intake, and bezafibrate treatment exerted no impact on these parameters. GC treatment caused an augmentation in fasting and fed glycemia, plasma triacylglycerol and nonesterified fatty acids, and insulinemia, and bezafibrate treatment completely prevented the elevation in plasma triacylglycerol and attenuated all other parameters. Insulin resistance and glucose intolerance induced by GC treatment were abolished and attenuated, respectively, by bezafibrate treatment.
Conclusion: High-dose bezafibrate treatment prevents the increase in plasma triacylglycerol and the development of insulin resistance and attenuates glucose intolerance in rats caused by GC treatment, indicating the involvement of dyslipidemia in the GC-induced insulin resistance.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

