Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke
- PMID: 30533170
- PMCID: PMC6250005
- DOI: 10.1155/2018/3172480
Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke
Abstract
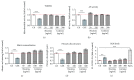
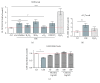
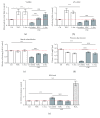
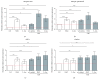
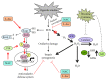
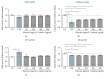
Cigarette smoking has been identified as a major risk factor for osteoporosis decades ago. Several studies have shown a direct relationship between cigarette smoking, decreased bone mineral density, and impaired fracture healing. However, the mechanisms behind impaired fracture healing and cigarette smoking are yet to be elucidated. Migration and osteogenesis of mesenchymal stem/stromal cells (MSCs) into the fracture site play a vital role in the process of fracture healing. In human nicotine, the most pharmacologically active and major addictive component present in tobacco gets rapidly metabolized to the more stable cotinine. This study demonstrates that physiological concentrations of both nicotine and cotinine do not affect the osteogenic differentiation of MSCs. However, cigarette smoke exposure induces oxidative stress by increasing superoxide radicals and reducing intracellular glutathione in MSCs, negatively affecting osteogenic differentiation. Although, not actively producing reactive oxygen species (ROS) nicotine and cotinine inhibit catalase and glutathione reductase activity, contributing to an accumulation of ROS by cigarette smoke exposure. Coincubation with N-acetylcysteine or L-ascorbate improves impaired osteogenesis caused by cigarette smoke exposure by both activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling and scavenging of ROS, which thus might represent therapeutic targets to support fracture healing in smokers.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

