Central masked adjudication of stroke diagnosis at trial entry offered no advantage over diagnosis by local clinicians: Secondary analysis and simulation
- PMID: 30533551
- PMCID: PMC6249966
- DOI: 10.1016/j.conctc.2018.11.002
Central masked adjudication of stroke diagnosis at trial entry offered no advantage over diagnosis by local clinicians: Secondary analysis and simulation
Abstract
Background: Central adjudication of stroke type is commonly implemented in large multicentre clinical trials. We investigated the effect of central adjudication of diagnosis of stroke type at trial entry in the Efficacy of Nitric Oxide in Stroke (ENOS) trial.
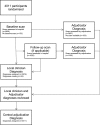
Methods: ENOS recruited patients with acute ischaemic or haemorrhagic stroke, and diagnostic adjudication was carried out using cranial scans. For this study, diagnoses made by local site clinicians were compared with those by central, masked adjudicators using kappa statistics. The trial primary analysis and subgroup analysis by stroke type were re-analysed using stroke diagnosis made by local clinicians, and simulations were used to assess the impact of increased non-differential misclassification and subgroup effects.
Results: Agreement on stroke type (Ischaemic, Intracerebral Haemorrhage, Unknown stroke type, No-stroke) was high (κ = 0.92). Adjudication of stroke type had no impact on the primary outcome or subgroup analysis by stroke type. With misclassification increased to 10 times the level observed in ENOS and a simulated subgroup effect present, adjudication would have affected trial conclusions.
Conclusions: Stroke type at trial entry was diagnosed accurately by local clinicians in ENOS. Adjudication of stroke type by central adjudicators had no measurable effect on trial conclusions. Diagnostic adjudication may be important if diagnosis is complex and a treatment-diagnosis interaction is expected.
Keywords: Adjudication; Clinical trial; Diagnosis; Stroke.
Figures
References
-
- Walter S.D., Cook D.J., Guyatt G.H., King D. Outcome assessment for clinical trials: how many adjudicators do we need? Contr. Clin. Trials. 1997;18(1):27–42. - PubMed
-
- Granger C.B., Vogel V., Cummings S.R., Held P., Fiedorek F., Lawrence M. Do we need to adjudicate major clinical events? Clin. Trials. 2008;5(1):56–60. - PubMed
-
- Pogue J., Walter S.D., Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clin. Trials. 2009;6(3):239–251. - PubMed
-
- Stuck A.K., Fuhrer E., Limacher A., Méan M., Aujesky D. Adjudication-related processes are underreported and lack standardization in clinical trials of venous thromboembolism: a systematic review. J. Clin. Epidemiol. 2014;67(3):278–284. - PubMed
-
- Dechartres A., Boutron I., Roy C., Ravaud P. Inadequate planning and reporting of adjudication committees in clinical trials: recommendation proposal. J. Clin. Epidemiol. 2009;62(7):695–702. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources