Exploratory evaluation of pharmacodynamics, pharmacokinetics and safety of rivaroxaban in children and adolescents: an EINSTEIN-Jr phase I study
- PMID: 30534007
- PMCID: PMC6278122
- DOI: 10.1186/s12959-018-0186-0
Exploratory evaluation of pharmacodynamics, pharmacokinetics and safety of rivaroxaban in children and adolescents: an EINSTEIN-Jr phase I study
Abstract
Background: The EINSTEIN-Jr program will evaluate rivaroxaban for the treatment of venous thromboembolism (VTE) in children, targeting exposures similar to the 20 mg once-daily dose for adults.
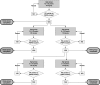
Methods: This was a multinational, single-dose, open-label, phase I study to describe the pharmacodynamics (PD), pharmacokinetics (PK) and safety of a single bodyweight-adjusted rivaroxaban dose in children aged 0.5-18 years. Children who had completed treatment for a venous thromboembolic event were enrolled into four age groups (0.5-2 years, 2-6 years, 6-12 years and 12-18 years) receiving rivaroxaban doses equivalent to 10 mg or 20 mg (either as a tablet or oral suspension). Blood samples for PK and PD analyses were collected within specified time windows.
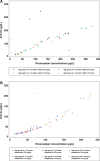
Results: Fifty-nine children were evaluated. In all age groups, PD parameters (prothrombin time, activated partial thromboplastin time and anti-Factor Xa activity) showed a linear relationship versus rivaroxaban plasma concentrations and were in line with previously acquired adult data, as well as in vitro spiking experiments. The rivaroxaban pediatric physiologically based pharmacokinetic model, used to predict the doses for the individual body weight groups, was confirmed. No episodes of bleeding were reported, and treatment-emergent adverse events occurred in four children and all resolved during the study.
Conclusions: Bodyweight-adjusted, single-dose rivaroxaban had predictable PK/PD profiles in children across all age groups from 0.5 to 18 years. The PD assessments based on prothrombin time and activated partial thromboplastin time demonstrated that the anticoagulant effect of rivaroxaban was not affected by developmental hemostasis in children.
Trial registration: ClinicalTrials.gov number, NCT01145859.
Keywords: Developmental hemostasis; Pharmacodynamics; Pharmacokinetics; Rivaroxaban; Venous thromboembolism.
Conflict of interest statement
The protocol was approved by the Institutional Review Board or Ethics Committee of each participating center, if required, and de-identified data was retrieved.Not applicable.DK, SW, MB, KT and AWAL are employees of Bayer. GY, AC, PS, and PM have no competing to report. LRB has received research funds from Sanofi Aventis, and has received honoraria from Bayer and from Boehringer Ingelheim. CM has received honoraria from Bayer AG, Boehringer Ingelheim, and Bristol-Myers Squibb. GK has received honoraria from OPKO biologics, Bayer, Pfizer, CSL, Alnylam, Shire and Roche. IM has received consultancy fees from Bayer.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Prins MH, Lensing AWA, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11:21. doi: 10.1186/1477-9560-11-21. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

