Studies of human hemoglobin modified with peroxynitrite: A cytotoxic metabolite generated in numerous disorders
- PMID: 30534041
- PMCID: PMC6257874
Studies of human hemoglobin modified with peroxynitrite: A cytotoxic metabolite generated in numerous disorders
Abstract
Objectives: Peroxynitrite interacts with biomolecules through oxidative reactions or radical-mediated mechanisms leading to oxidative damage and committing cells to necrosis or/and apoptosis. Hemoglobin (Hb) is the oxygen-transporting metalloprotein found in blood that carries oxygen from the lungs to the tissues and subsequently releases it to carry out various metabolic functions. In the present study, we have isolated Hb from human blood and subjected it to modify by peroxynitrite generated in vitro. The native and modified Hbs were characterized using various biochemical methods.
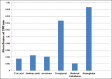
Methods: The native and modified Hbs were characterized using absorption spectroscopy, thermal melting profile analysis, and other biochemical techniques. We have also tried to ascertain the effect of various scavengers such as uric acid, ascorbic acid, tocopherol, and reduced glutathione as potent peroxynitrite quenchers.
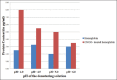
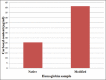
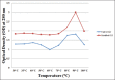
Results: The isolated Hb produces distinct peaks while the Hb modified with peroxynitrite showed marked hyperchromicity and the distinct peaks were lost. The chemical denaturation and thermal denaturation studies along with carbonyl content data show that the modified Hb is unstable and shows higher absorbance due to denaturation of the protein.
Conclusion: Thus, the formation and effect of peroxynitrite on Hb are deleterious and antioxidant scavengers of the peroxynitrite show that the modification of the Hb can reverse the effect of peroxynitrite modification. The in vitro studies presented here show that peroxynitrite is toxic to human Hb and its inhibition by various antioxidants may be helpful in prevention of numerous disorders.
Keywords: Biochemical characterization; hemoglobin; peroxynitrite.
Figures
References
-
- Pittman RN. Regulation of Tissue Oxygenation. Vol. 4. San Rafael (CA): Morgan and Claypool Life Sciences; Oxygen Transport; 2016. pp. 23–35. - PubMed
-
- Pryor WA, Squadrito GL. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–722. - PubMed
LinkOut - more resources
Full Text Sources
