Leptin Functions in Infectious Diseases
- PMID: 30534129
- PMCID: PMC6275238
- DOI: 10.3389/fimmu.2018.02741
Leptin Functions in Infectious Diseases
Abstract
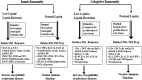
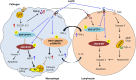
Leptin, a pleiotropic protein has long been recognized to play an important role in the regulation of energy homeostasis, metabolism, neuroendocrine function, and other physiological functions through its effects on the central nervous system (CNS) and peripheral tissues. Leptin is secreted by adipose tissue and encoded by the obese (ob) gene. Leptin acts as a central mediator which regulates immunity as well as nutrition. Importantly, leptin can modulate both innate and adaptive immune responses. Leptin deficiency/resistance is associated with dysregulation of cytokine production, increased susceptibility toward infectious diseases, autoimmune disorders, malnutrition and inflammatory responses. Malnutrition induces a state of immunodeficiency and an inclination to death from communicable diseases. Infectious diseases are the disease of poor who invariably suffer from malnutrition that could result from reduced serum leptin levels. Thus, leptin has been placed at the center of many interrelated functions in various pathogenic conditions, such as bacterial, viruses and parasitic infections. We review herein, the recent advances on the role of leptin in malnutrition in pathogenesis of infectious diseases with a particular emphasis on parasitic diseases such as Leishmaniasis, Trypanosomiasis, Amoebiasis, and Malaria.
Keywords: amoebiasis; bacteria; leishmaniasis; leptin; malaria; malnutrition; trypanosomiasis; virus.
Figures
References
-
- Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, et al. . Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. (1996) 81:3861–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

