Reproductive and Behavior Dysfunction Induced by Maternal Androgen Exposure and Obesity Is Likely Not Gut Microbiome-Mediated
- PMID: 30534630
- PMCID: PMC6280317
- DOI: 10.1210/js.2018-00266
Reproductive and Behavior Dysfunction Induced by Maternal Androgen Exposure and Obesity Is Likely Not Gut Microbiome-Mediated
Abstract
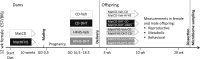
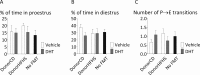
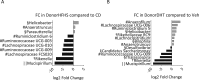
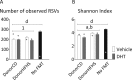
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder of unclear etiology in women and is characterized by androgen excess, insulin resistance, and mood disorders. The gut microbiome is known to influence conditions closely related with PCOS, and several recent studies have observed changes in the stool microbiome of women with PCOS. The mechanism by which the gut microbiome interacts with PCOS is still unknown. We used a mouse model to investigate if diet-induced maternal obesity and maternal DHT exposure, mimicking the lean and obese PCOS women, cause lasting changes in the gut microbiome of offspring. Fecal microbiome profiles were assessed using Illumina paired-end sequencing of 16S rRNA gene V4 amplicons. We found sex-specific effects of maternal and offspring diet, and maternal DHT exposure on fecal bacterial richness and taxonomic composition. Female offspring exposed to maternal obesity and DHT displayed reproductive dysfunction and anxietylike behavior. Fecal microbiota transplantation from DHT and diet-induced obesity exposed female offspring to wild-type mice did not transfer reproductive dysfunction and did not cause the expected increase in anxietylike behavior in recipients. Maternal obesity and androgen exposure affect the gut microbiome of offspring, but the disrupted estrous cycles and anxietylike behavior are likely not microbiome-mediated.
Keywords: androgens; anxiety; diet-induced obesity; gut microbiota; polycystic ovary syndrome.
Figures







Similar articles
-
The interplay between PCOS pathology and diet on gut microbiota in a mouse model.Gut Microbes. 2022 Jan-Dec;14(1):2085961. doi: 10.1080/19490976.2022.2085961. Gut Microbes. 2022. PMID: 35787106 Free PMC article.
-
Maternal androgen excess induces cardiac hypertrophy and left ventricular dysfunction in female mice offspring.Cardiovasc Res. 2020 Mar 1;116(3):619-632. doi: 10.1093/cvr/cvz180. Cardiovasc Res. 2020. PMID: 31382275
-
Intersection of Polycystic Ovary Syndrome and the Gut Microbiome.J Endocr Soc. 2020 Nov 16;5(2):bvaa177. doi: 10.1210/jendso/bvaa177. eCollection 2021 Feb 1. J Endocr Soc. 2020. PMID: 33381671 Free PMC article. Review.
-
Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring.FASEB J. 2018 Aug;32(8):4158-4171. doi: 10.1096/fj.201701263RR. Epub 2018 Mar 22. FASEB J. 2018. PMID: 29565738
-
A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota.J Am Coll Nutr. 2020 May-Jun;39(4):371-382. doi: 10.1080/07315724.2019.1657515. Epub 2019 Sep 12. J Am Coll Nutr. 2020. PMID: 31513473 Review.
Cited by
-
The Gut Microbiome and Female Health.Biology (Basel). 2022 Nov 21;11(11):1683. doi: 10.3390/biology11111683. Biology (Basel). 2022. PMID: 36421397 Free PMC article. Review.
-
Pathophysiology of polycystic ovary syndrome revisited: Current understanding and perspectives regarding future research.Reprod Med Biol. 2022 Oct 8;21(1):e12487. doi: 10.1002/rmb2.12487. eCollection 2022 Jan-Dec. Reprod Med Biol. 2022. PMID: 36310656 Free PMC article.
-
Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls.Gut Microbes. 2021 Jan-Dec;13(1):1941711. doi: 10.1080/19490976.2021.1941711. Gut Microbes. 2021. PMID: 34328058 Free PMC article. Review.
-
The interplay between PCOS pathology and diet on gut microbiota in a mouse model.Gut Microbes. 2022 Jan-Dec;14(1):2085961. doi: 10.1080/19490976.2022.2085961. Gut Microbes. 2022. PMID: 35787106 Free PMC article.
-
Maternal and early life exposures and their potential to influence development of the microbiome.Genome Med. 2022 Jan 11;14(1):4. doi: 10.1186/s13073-021-01005-7. Genome Med. 2022. PMID: 35016706 Free PMC article. Review.
References
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47. - PubMed
-
- Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149(5):R219–R227. - PubMed
-
- Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–1091. - PubMed
-
- Chan JL, Kar S, Vanky E, Morin-Papunen L, Piltonen T, Puurunen J, Tapanainen JS, Maciel GAR, Hayashida SAY, Soares JM Jr, Baracat EC, Mellembakken JR, Dokras A. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol. 2017;217(2):189.e1–189.e8. - PubMed
-
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
