Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy
- PMID: 30535360
- PMCID: PMC6452193
- DOI: 10.1093/hmg/ddy420
Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy
Erratum in
-
Corrigendum: Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy.Hum Mol Genet. 2019 May 15;28(10):1752. doi: 10.1093/hmg/ddz021. Hum Mol Genet. 2019. PMID: 30668730 Free PMC article. No abstract available.
-
Corrigendum: Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy.Hum Mol Genet. 2019 Jul 1;28(13):2282. doi: 10.1093/hmg/ddz037. Hum Mol Genet. 2019. PMID: 31220267 Free PMC article. No abstract available.
Abstract
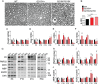
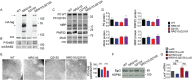
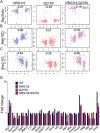
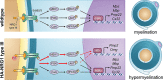
Myelin sheath thickness is precisely regulated and essential for rapid propagation of action potentials along myelinated axons. In the peripheral nervous system, extrinsic signals from the axonal protein neuregulin 1 (NRG1) type III regulate Schwann cell fate and myelination. Here we ask if modulating NRG1 type III levels in neurons would restore myelination in a model of congenital hypomyelinating neuropathy (CHN). Using a mouse model of CHN, we improved the myelination defects by early overexpression of NRG1 type III. Surprisingly, the improvement was independent from the upregulation of Egr2 or essential myelin genes. Rather, we observed the activation of MAPK/ERK and other myelin genes such as peripheral myelin protein 2 and oligodendrocyte myelin glycoprotein. We also confirmed that the permanent activation of MAPK/ERK in Schwann cells has detrimental effects on myelination. Our findings demonstrate that the modulation of axon-to-glial NRG1 type III signaling has beneficial effects and improves myelination defects during development in a model of CHN.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Enhanced axonal neuregulin-1 type-III signaling ameliorates neurophysiology and hypomyelination in a Charcot-Marie-Tooth type 1B mouse model.Hum Mol Genet. 2019 Mar 15;28(6):992-1006. doi: 10.1093/hmg/ddy411. Hum Mol Genet. 2019. PMID: 30481294 Free PMC article.
-
Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function.J Neurosci. 2006 Mar 22;26(12):3079-86. doi: 10.1523/JNEUROSCI.3785-05.2006. J Neurosci. 2006. PMID: 16554459 Free PMC article.
-
Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination.J Neurosci. 2010 Apr 28;30(17):6122-31. doi: 10.1523/JNEUROSCI.1681-09.2010. J Neurosci. 2010. PMID: 20427670 Free PMC article.
-
New insights into signaling during myelination in zebrafish.Curr Top Dev Biol. 2011;97:1-19. doi: 10.1016/B978-0-12-385975-4.00007-3. Curr Top Dev Biol. 2011. PMID: 22074600 Free PMC article. Review.
-
Neuregulin/ErbB Signaling in Developmental Myelin Formation and Nerve Repair.Curr Top Dev Biol. 2016;116:45-64. doi: 10.1016/bs.ctdb.2015.11.009. Epub 2016 Feb 1. Curr Top Dev Biol. 2016. PMID: 26970613 Review.
Cited by
-
High-Fat Diet Disrupt Nerve Function by Targeting Schwann Cells.J Peripher Nerv Syst. 2025 Jun;30(2):e70036. doi: 10.1111/jns.70036. J Peripher Nerv Syst. 2025. PMID: 40522084
-
A new mouse model of Charcot-Marie-Tooth 2J neuropathy replicates human axonopathy and suggest alteration in axo-glia communication.PLoS Genet. 2022 Nov 9;18(11):e1010477. doi: 10.1371/journal.pgen.1010477. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36350884 Free PMC article.
-
Type III NRG-1 plays a regulatory role in the regeneration process of nerves from the beginning of transplantation.J Orthop Surg Res. 2023 Sep 20;18(1):707. doi: 10.1186/s13018-023-04191-9. J Orthop Surg Res. 2023. PMID: 37730632 Free PMC article.
-
Cognitive Impairments Related to COMT and Neuregulin 1 Phenotypes as Transdiagnostic Markers in Schizophrenia Spectrum Patients.J Clin Med. 2024 Oct 25;13(21):6405. doi: 10.3390/jcm13216405. J Clin Med. 2024. PMID: 39518545 Free PMC article.
-
Schwann cell interactions during the development of the peripheral nervous system.Dev Neurobiol. 2021 Jul;81(5):464-489. doi: 10.1002/dneu.22744. Epub 2020 May 5. Dev Neurobiol. 2021. PMID: 32281247 Free PMC article. Review.
References
-
- Meyer D. and Birchmeier C. (1995) Multiple essential functions of neuregulin in development. Nature, 378, 386–390. - PubMed
-
- Riethmacher D., Sonnenberg-Riethmacher E., Brinkmann V., Yamaai T., Lewin G.R. and Birchmeier C. (1997) Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature, 389, 725–730. - PubMed
-
- Birchmeier C. and Nave K.A. (2008) Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia, 56, 1491–1497. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

