Delegating Sex: Differential Gene Expression in Stolonizing Syllids Uncovers the Hormonal Control of Reproduction
- PMID: 30535381
- PMCID: PMC6350857
- DOI: 10.1093/gbe/evy265
Delegating Sex: Differential Gene Expression in Stolonizing Syllids Uncovers the Hormonal Control of Reproduction
Abstract
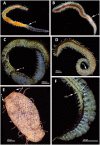
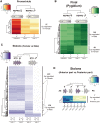
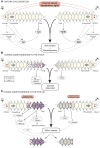
Stolonization in syllid annelids is a unique mode of reproduction among animals. During the breeding season, a structure resembling the adult but containing only gametes, called stolon, is formed generally at the posterior end of the animal. When stolons mature, they detach from the adult and gametes are released into the water column. The process is synchronized within each species, and it has been reported to be under environmental and endogenous control, probably via endocrine regulation. To further understand reproduction in syllids and to elucidate the molecular toolkit underlying stolonization, we generated Illumina RNA-seq data from different tissues of reproductive and nonreproductive individuals of Syllis magdalena and characterized gene expression during the stolonization process. Several genes involved in gametogenesis (ovochymase, vitellogenin, testis-specific serine/threonine-kinase), immune response (complement receptor 2), neuronal development (tyrosine-protein kinase Src42A), cell proliferation (alpha-1D adrenergic receptor), and steroid metabolism (hydroxysteroid dehydrogenase 2) were found differentially expressed in the different tissues and conditions analyzed. In addition, our findings suggest that several neurohormones, such as methyl farnesoate, dopamine, and serotonin, might trigger stolon formation, the correct maturation of gametes and the detachment of stolons when gametogenesis ends. The process seems to be under circadian control, as indicated by the expression patterns of r-opsins. Overall, our results shed light into the genes that orchestrate the onset of gamete formation and improve our understanding of how some hormones, previously reported to be involved in reproduction and metamorphosis processes in other invertebrates, seem to also regulate reproduction via stolonization.
Figures







References
-
- Abascal F, Zardoya R, Posada D.. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21(9):2104–2105. - PubMed
-
- Abeloos M. 1950. Régénération et stolonisation épigame chez l’Annélide Syllis prolifera Krohn. C R Acad Sci (Comptes rendus de l'Academie des Sciences). 230:1899–1900. - PubMed
-
- Agassiz A. 1863. On alternate generation in annelids, and the embryology of Autolytus cornutus. Boston J Nat Hist. 7:384–409.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

