Understanding the importance of low-molecular weight (ethylene oxide- and propylene oxide-induced) DNA adducts and mutations in risk assessment: Insights from 15 years of research and collaborative discussions
- PMID: 30536466
- PMCID: PMC6590209
- DOI: 10.1002/em.22248
Understanding the importance of low-molecular weight (ethylene oxide- and propylene oxide-induced) DNA adducts and mutations in risk assessment: Insights from 15 years of research and collaborative discussions
Abstract
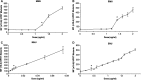
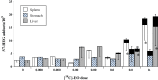
The interpretation and significance of DNA adduct data, their causal relationship to mutations, and their role in risk assessment have been debated for many years. An extended effort to identify key questions and collect relevant data to address them was focused on the ubiquitous low MW N7-alkyl/hydroxyalkylguanine adducts. Several academic, governmental, and industrial laboratories collaborated to gather new data aimed at better understanding the role and potential impact of these adducts in quantifiable genotoxic events (gene mutations/micronucleus). This review summarizes and evaluates the status of dose-response data for DNA adducts and mutations from recent experimental work with standard mutagenic agents and ethylene oxide and propylene oxide, and the importance for risk assessment. This body of evidence demonstrates that small N7-alkyl/hydroxyalkylguanine adducts are not pro-mutagenic and, therefore, adduct formation alone is not adequate evidence to support a mutagenic mode of action. Quantitative methods for dose-response analysis and derivation of thresholds, benchmark dose (BMD), or other points-of-departure (POD) for genotoxic events are now available. Integration of such analyses of genetox data is necessary to properly assess any role for DNA adducts in risk assessment. Regulatory acceptance and application of these insights remain key challenges that only the regulatory community can address by applying the many learnings from recent research. The necessary tools, such as BMDs and PODs, and the example datasets, are now available and sufficiently mature for use by the regulatory community. Environ. Mol. Mutagen. 60: 100-121, 2019. © 2018 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Keywords: DNA adducts; N7-alkyl/hydroxyalkylguanine adducts; dose-response; genotoxic effects; mutations.
© 2018 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Figures
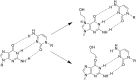




 Exogenous Compounds Requiring Metabolic Activation.
Exogenous Compounds Requiring Metabolic Activation.  Exogenous Reactive Direct‐Acting Compounds.
Exogenous Reactive Direct‐Acting Compounds.  Endogenous Reactive Direct‐Acting Compounds.
Endogenous Reactive Direct‐Acting Compounds.
Similar articles
-
Formation of DNA adducts and induction of mutagenic effects in rats following 4 weeks inhalation exposure to ethylene oxide as a basis for cancer risk assessment.Mutat Res. 2000 Jan 17;447(1):27-48. doi: 10.1016/s0027-5107(99)00208-0. Mutat Res. 2000. PMID: 10686305
-
Genotoxic effects of inhaled ethylene oxide, propylene oxide and butylene oxide on germ cells: sensitivity of genetic endpoints in relation to dose and repair status.Mutat Res. 1998 Sep 20;405(2):259-71. doi: 10.1016/s0027-5107(98)00143-2. Mutat Res. 1998. PMID: 9748619
-
Ethylene oxide and propylene oxide derived N7-alkylguanine adducts are bypassed accurately in vivo.DNA Repair (Amst). 2014 Oct;22:133-6. doi: 10.1016/j.dnarep.2014.08.001. Epub 2014 Aug 30. DNA Repair (Amst). 2014. PMID: 25173234
-
Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: update review (1990-2001).Mutat Res. 2002 Dec;512(2-3):173-94. doi: 10.1016/s1383-5742(02)00067-4. Mutat Res. 2002. PMID: 12464351 Review.
-
Carcinogenicity and genotoxicity of ethylene oxide: new aspects and recent advances.Crit Rev Toxicol. 2000 Sep;30(5):595-608. doi: 10.1080/10408440008951121. Crit Rev Toxicol. 2000. PMID: 11055837 Review.
Cited by
-
Biological Basis for Threshold Responses to Methylating Agents.Chem Res Toxicol. 2020 Sep 21;33(9):2219-2224. doi: 10.1021/acs.chemrestox.0c00052. Epub 2020 May 27. Chem Res Toxicol. 2020. PMID: 32388971 Free PMC article. Review.
-
Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk.Foods. 2022 Sep 13;11(18):2828. doi: 10.3390/foods11182828. Foods. 2022. PMID: 36140952 Free PMC article. Review.
-
Current and Future Methodology for Quantitation and Site-Specific Mapping the Location of DNA Adducts.Toxics. 2022 Jan 19;10(2):45. doi: 10.3390/toxics10020045. Toxics. 2022. PMID: 35202232 Free PMC article.
-
The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment.Arch Toxicol. 2022 May;96(5):1297-1352. doi: 10.1007/s00204-022-03242-0. Epub 2022 Mar 6. Arch Toxicol. 2022. PMID: 35249149 Free PMC article. Review.
-
Critical review of styrene genotoxicity focused on the mutagenicity/clastogenicity literature and using current organization of economic cooperation and development guidance.Environ Mol Mutagen. 2019 Aug;60(7):624-663. doi: 10.1002/em.22278. Epub 2019 Mar 13. Environ Mol Mutagen. 2019. PMID: 30786062 Free PMC article. Review.
References
-
- Aramini JM, Tubbs JL, Kanugula S, Rossi P, Ertekin A, Maglaqui M, Hamilton K, Ciccosanti CT, Jiang M, Xiao R, Soong TT, Rost B, Acton TB, Everett JK, Pegg AE, Tainer JA, Montelione GT. 2010. Structural basis of O6‐alkylguanine recognition by a bacterial alkyltransferase‐like DNA repair protein. J Biol Chem 285(18):13736–13741. - PMC - PubMed
-
- Barnes DE, Lindahl T. 2004. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Ann Rev Genetics 38:445–476. - PubMed
-
- Bartsch H. 1996. DNA adducts in human carcinogenesis: Etiological relevance and structure‐activity relationship. Mutat Res/Rev Genetic Toxicol 340(2–3):67–79. - PubMed
-
- Beranek DT. 1990. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res 231(1):11–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

